Pandemic influenza vaccines Viral vaccines in the medical











- Slides: 17

Pandemic influenza vaccines Viral vaccines in the medical practice 8 June 2010, Cluj-Napoca Kálmán Bartha Ph. D Zsuzsanna Pauliny MD bartha. kalman@oek. antsz. hu zsuzsanna. pauliny@oek. antsz. hu National Centre for Epidemiology Budapest, Hungary

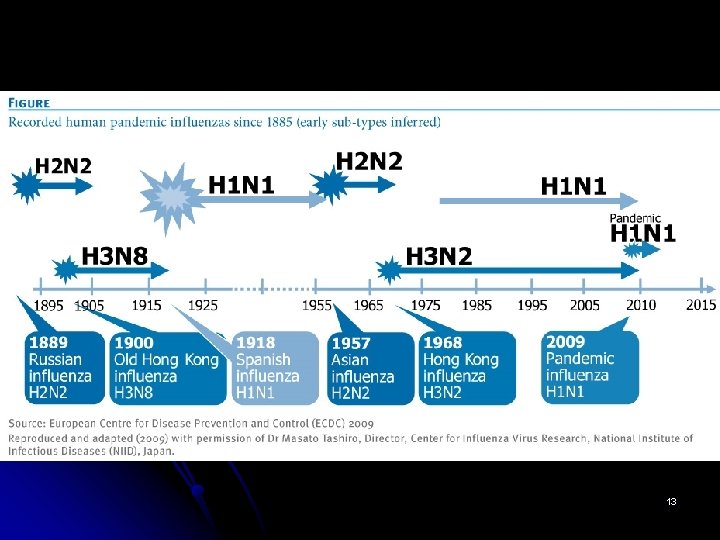
Flu – benign or devastating? l l l Though widespread familiarity with the flu makes it seem relatively benign to much of the general population, the virus can be devastating. In 1918 and 1919, more than 20 million people died from a strain of the virus commonly known as the Spanish flu that circulated through almost all inhabited regions of the globe. Many other outbreaks have occurred since that time, though none have been as deadly. Nevertheless, influenza together with complications of the virus is consistently among the top ten common causes of death in the World, ranking higher than some other much more widely publicized killers, such as the HIV virus that causes AIDS. 2

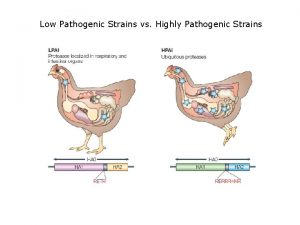
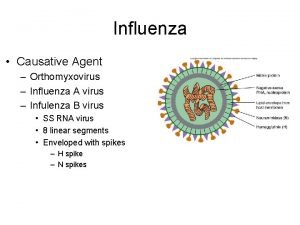
Influenza viruses are normally highly species specific l l l As influenza viruses are normally highly species specific, they only rarely spill over to cause infection in other species. This is due to differences in the use of cellular receptors. Avian influenza viruses bind to cell-surface glycoproteins containing sialyl-galactosyl residues linked by a 2 -3 -linkage, whereas human viruses bind to receptors that contain terminal 2 -6 -linked sialylgalactosyl moieties. For an avian virus to be easily transmitted between humans, it is fundamental that it acquires the ability to bind cells that display the 2 -6 receptors so that it can enter the cell and replicate in them. While single amino acid substitutions can significantly alter receptor specificity of avian H 5 N 1 viruses, human infections with avian influenza viruses have only rarely occurred. Of the hundreds of strains of avian influenza A viruses, only four are known to have caused human infection: H 5 N 1, H 7 N 3, H 7 N 7, and H 9 N 2. Apart from H 5 N 1, human infection generally resulted in mild symptoms and rarely in severe illness. 3

4

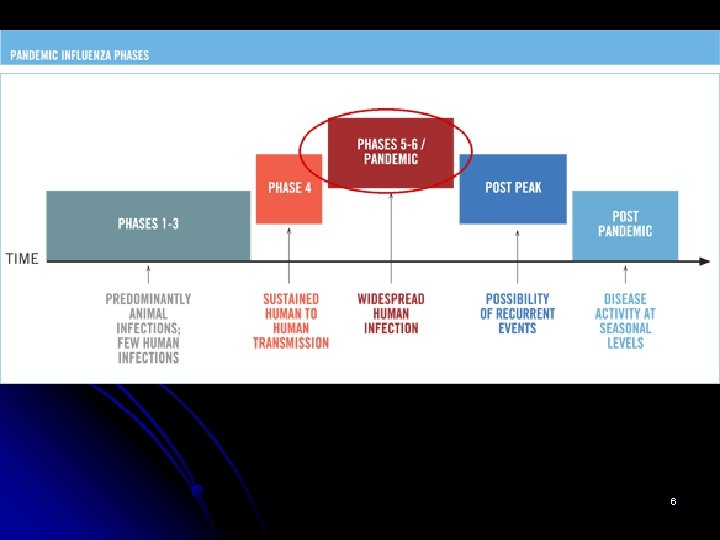
WHO phases of pandemic alert l l l Pandemic preparedness In the 2009 revision of the phase descriptions, WHO has retained the use of a six-phased approach for easy incorporation of new recommendations and approaches into existing national preparedness and response plans. The grouping and description of pandemic phases have been revised to make them easier to understand, more precise, and based upon observable phenomena. Phases 1– 3 correlate with preparedness, including capacity development and response planning activities, while Phases 4– 6 clearly signal the need for response and mitigation efforts. Furthermore, periods after the first pandemic wave are elaborated to facilitate post pandemic recovery activities. 5 The current WHO phase of pandemic alert is 6.

6

l l In nature, influenza viruses circulate continuously among animals, especially birds. Even though such viruses might theoretically develop into pandemic viruses, In Phase 1 no viruses circulating among animals have been reported to cause infections in humans. In Phase 2 an animal influenza virus circulating among domesticated or wild animals is known to have caused infection in humans, and is therefore considered a potential pandemic threat. In Phase 3 an animal or human-animal influenza reassortant virus has caused sporadic cases or small clusters of disease in people, but has not resulted in human-to-human transmission sufficient to sustain community-level outbreaks. Limited human-to-human transmission may occur under some circumstances, for example, when there is close contact between an infected person and an unprotected caregiver. However, limited transmission under such restricted circumstances does not indicate that the virus has gained the level of transmissibility among humans necessary to cause a 7 pandemic.

l l l Phase 4 is characterized by verified human-to-human transmission of an animal or human-animal influenza reassortant virus able to cause “community-level outbreaks. ” The ability to cause sustained disease outbreaks in a community marks a significant upwards shift in the risk for a pandemic. Any country that suspects or has verified such an event should urgently consult with WHO so that the situation can be jointly assessed and a decision made by the affected country if implementation of a rapid pandemic containment operation is warranted. Phase 4 indicates a significant increase in risk of a pandemic but does not necessarily mean that a pandemic is a forgone conclusion. Phase 5 is characterized by human-to-human spread of the virus into at least two countries in one WHO region. While most countries will not be affected at this stage, the declaration of Phase 5 is a strong signal that a pandemic is imminent and that the time to finalize the organization, communication, and implementation of the planned mitigation measures is short. Phase 6, the pandemic phase, is characterized by community level outbreaks in at least one other country in a different WHO region in addition to the criteria defined in Phase 5. Designation of this phase will indicate that a global pandemic is under way. 8

l l During the post-peak period, pandemic disease levels in most countries with adequate surveillance will have dropped below peak observed levels. The post-peak period signifies that pandemic activity appears to be decreasing; however, it is uncertain if additional waves will occur and countries will need to be prepared for a second wave. Previous pandemics have been characterized by waves of activity spread over months. Once the level of disease activity drops, a critical communications task will be to balance this information with the possibility of another wave. Pandemic waves can be separated by months and an immediate “at-ease” signal may be premature. In the post-pandemic period, influenza disease activity will have returned to levels normally seen for seasonal influenza. It is expected that the pandemic virus will behave as a seasonal influenza A virus. At this stage, it is important to maintain surveillance and update pandemic preparedness and response plans accordingly. An intensive phase of recovery and evaluation may be required. 9

10

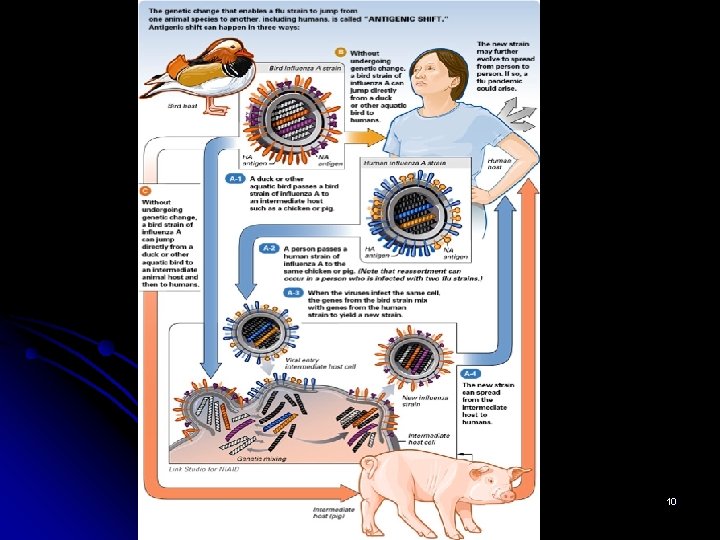
Antigenic shift l l Influenza A experiences a special type of mutation called antigenic shift that results in a new subtype of the virus. Antigenic shift is a sudden change in antigenicity caused by the recombination of the influenza genome, which can occur when a cell becomes simultaneously infected by two different strains of type A influenza. The unusually broad range of hosts susceptible to influenza A appears to increase the likelihood that this event will occur. In particular, the mixing of strains that can infect birds, pigs, and humans is thought to be responsible for most antigenic shifts. Notably, in some parts of the world, humans live in close proximity to both swine and fowl, so that human strains and bird strains, may readily infect a pig at the same time, resulting in a unique virus. l New subtypes of influenza A develop abruptly and unpredictably so that scientists are unable to prepare vaccines in advance that are effective against them. Consequently, the emergence of a new subtype of the virus can cause a global pandemic in a very short amount of time. 11

12

13

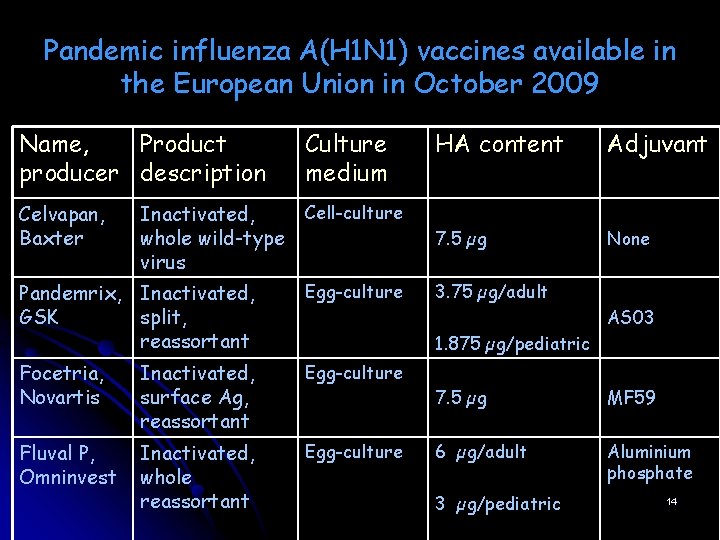
Pandemic influenza A(H 1 N 1) vaccines available in the European Union in October 2009 Name, Product producer description Culture medium Celvapan, Baxter Cell-culture Inactivated, whole wild-type virus Pandemrix, Inactivated, GSK split, reassortant Egg-culture Focetria, Novartis Inactivated, surface Ag, reassortant Egg-culture Fluval P, Omninvest Inactivated, whole reassortant Egg-culture HA content Adjuvant 7. 5 µg None 3. 75 µg/adult AS 03 1. 875 µg/pediatric 7. 5 µg MF 59 6 µg/adult Aluminium phosphate 3 µg/pediatric 14

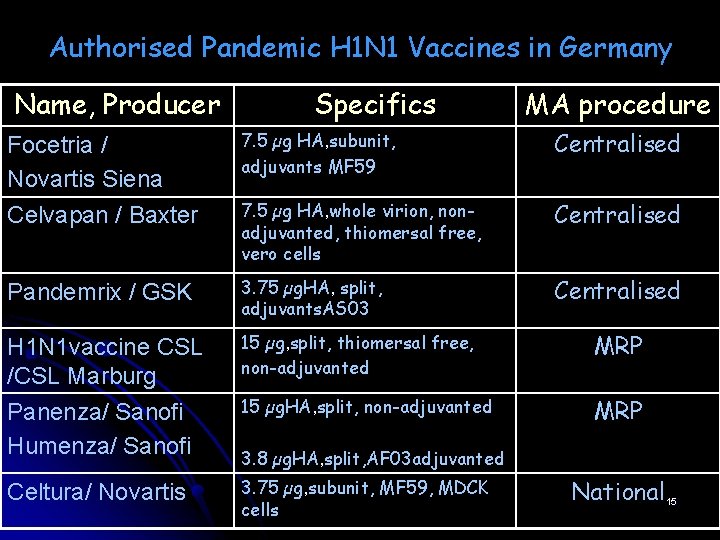
Authorised Pandemic H 1 N 1 Vaccines in Germany Name, Producer Specifics MA procedure Focetria / Novartis Siena Celvapan / Baxter 7. 5 µg HA, subunit, adjuvants MF 59 Centralised 7. 5 µg HA, whole virion, nonadjuvanted, thiomersal free, vero cells Centralised Pandemrix / GSK 3. 75 µg. HA, split, adjuvants. AS 03 Centralised H 1 N 1 vaccine CSL /CSL Marburg Panenza/ Sanofi Humenza/ Sanofi 15 µg, split, thiomersal free, non-adjuvanted MRP 15 µg. HA, split, non-adjuvanted MRP Celtura/ Novartis 3. 75 µg, subunit, MF 59, MDCK cells 3. 8 µg. HA, split, AF 03 adjuvanted National 15

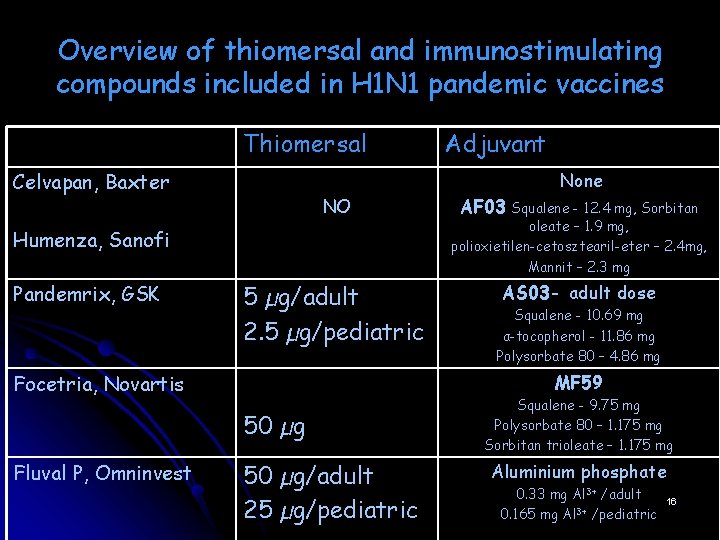
Overview of thiomersal and immunostimulating compounds included in H 1 N 1 pandemic vaccines Thiomersal Celvapan, Baxter None NO Squalene - 12. 4 mg, Sorbitan oleate – 1. 9 mg, polioxietilen-cetosztearil-eter – 2. 4 mg, Mannit – 2. 3 mg 5 µg/adult 2. 5 µg/pediatric AS 03 - adult dose Humenza, Sanofi Pandemrix, GSK Focetria, Novartis AF 03 Squalene - 10. 69 mg α-tocopherol - 11. 86 mg Polysorbate 80 – 4. 86 mg MF 59 50 µg Fluval P, Omninvest Adjuvant 50 µg/adult 25 µg/pediatric Squalene - 9. 75 mg Polysorbate 80 – 1. 175 mg Sorbitan trioleate – 1. 175 mg Aluminium phosphate 0. 33 mg Al 3+ /adult 0. 165 mg Al 3+ /pediatric 16

Antiviral medications l l In addition to vaccines, a few other weapons have been designed to combat the flu. The antiviral medications amantadine and rimantadine can help reduce severity of illness in individuals with influenza that begin utilizing the drugs within two days of the onset of symptoms. These drugs work by hindering the change in p. H that is necessary for the flu virion to release its contents into the cytosol of a host cell. Two additional antiviral drugs, zanamavir and oseltamivir, are effective against both A and B types of influenza. Instead of interfering with p. H shifts, zanamavir and oseltamivir block the glycoprotein neuraminidase so that the release of new virus particles is inhibited and their spread is thwarted. It is important to note that antibiotics are not capable of fighting the influenza virus itself, but are sometimes given to patients with the flu to stem attacks of opportunistic microorganisms that are responsible for many influenza complications. 17
 Mathalicious pandemic answer key
Mathalicious pandemic answer key Covid 19 pandemic summary
Covid 19 pandemic summary Pip framework
Pip framework Interpandemic period
Interpandemic period Pandemic tabletop exercise template
Pandemic tabletop exercise template Stomach flu vs influenza
Stomach flu vs influenza Influenza virus replication
Influenza virus replication Influenza ww1
Influenza ww1 The great influenza rhetorical analysis
The great influenza rhetorical analysis Is influenza a airborne disease
Is influenza a airborne disease Low pathogenic avian influenza
Low pathogenic avian influenza Albert osterhaus
Albert osterhaus Rimantidina
Rimantidina Piano di divisione delle staffe
Piano di divisione delle staffe Influenza
Influenza Influenza vaccine dosage chart 2019-2020
Influenza vaccine dosage chart 2019-2020 Causative agent of influenza
Causative agent of influenza Causes of viral hemorrhagic fever
Causes of viral hemorrhagic fever