Seasonal influenza vaccines Viral vaccines in the medical
























- Slides: 28

Seasonal influenza vaccines Viral vaccines in the medical practice 8 June 2010, Cluj-Napoca Kálmán Bartha Ph. D Zsuzsanna Pauliny MD bartha. kalman@oek. antsz. hu zsuzsanna. pauliny@oek. antsz. hu National Centre for Epidemiology Budapest, Hungary

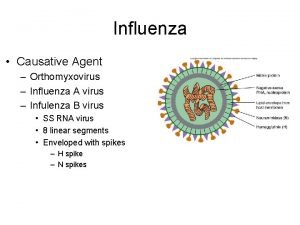
Types of influenza viruses l l l Three distinct types of influenza virus A, B, and C, have been identified. These viruses, which are antigenically distinct from one another, comprise their own viral family, Orthomyxoviridae. Most cases of the flu, especially those that occur in epidemics or pandemics, are caused by the influenza A virus, which can infect a variety of animal species too. The B virus, which normally is only found in humans, is responsible for many localized outbreaks. The C virus is morphologically and genetically different from the other two viruses and is generally nonsymptomatic, so it has a little medical concern. 2

3

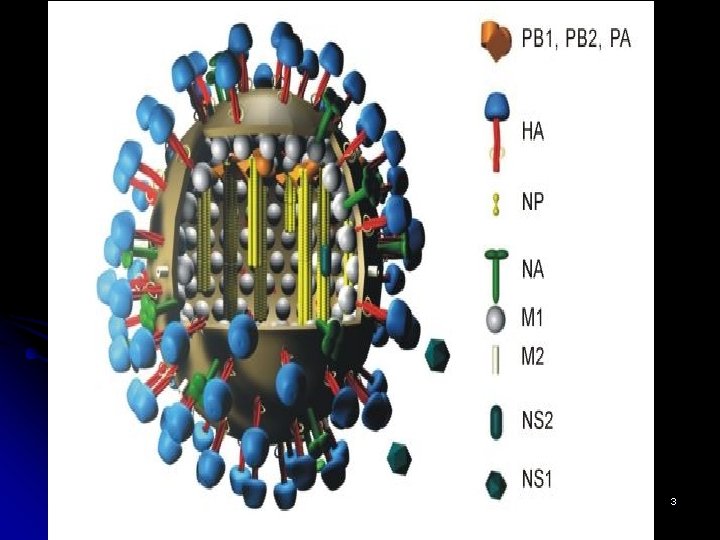
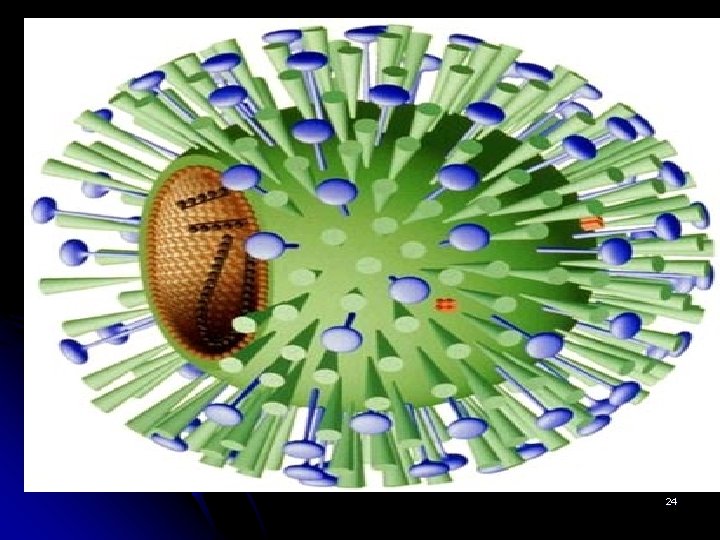
The structure of the influenza virus l l l The structure of the influenza virus is somewhat variable, but the virion particles are usually spherical or ovoid in shape and 80 to 120 nanometers in diameter. Sometimes filamentous forms of the virus occur as well. The influenza virion is an enveloped virus that derives its lipid bilayer from the plasma membrane of a host cell. Two different glycoprotein spikes are in the envelope. The haemagglutinin is approximately 80 percent of the spikes, it is a trimeric protein and the function is the attachment of the virus to a host cell. The remaining 20 percent of the glycoprotein spikes consist of neuraminidase, which is predominantly involved in facilitating the release of newly produced virus particles from the host cell. On the inner side of the envelope that surrounds an influenza virion is an antigenic matrix protein lining. Within the envelope is the influenza genome, which is organized into eight pieces of singlestranded RNA (A and B forms only; influenza C has 7 RNA segments). The RNA is packaged with nucleoprotein into a helical ribonucleoprotein form, with three polymerase peptides for each RNA segment. 4

5

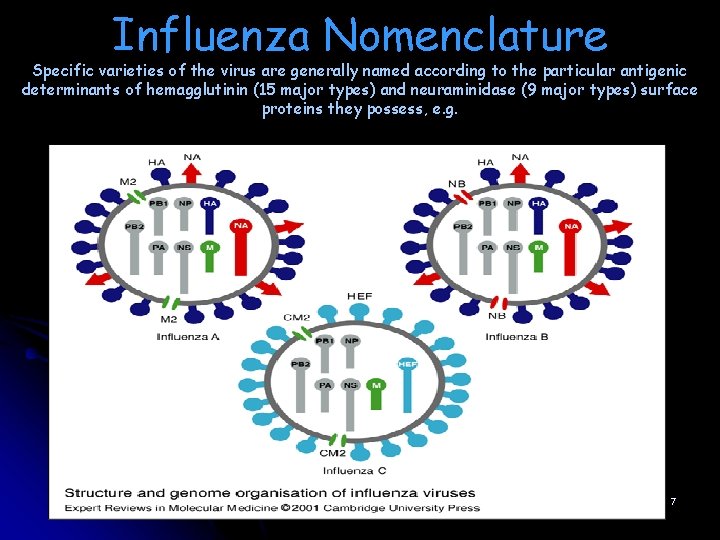
Structure and genome organisation of influenza viruses. l l The surface proteins of each virus and their respective genes are shown in colour (blue, red and green); other genes are shown in light grey. The interior proteins, namely the matrix protein (M 1), the nucleoprotein (NP) and the polymerases are not shown. Influenza A and B viruses contain eight RNA segments (genes), whereas influenza C viruses contain only seven RNA segments. Influenza C viruses contain a single surface glycoprotein (the haemagglutinin-esterase-fusion, or HEF, glycoprotein; shown in light blue), which functionally replaces the two surface glycoproteins that are found in influenza A and B viruses, namely haemagglutinin HA and neuraminidase NA. The envelopes of the three viruses also contain different ion channels, which are encoded by either the M gene (i. e. M 2 or CM 2, shown as green ovals) or the NA gene (i. e. NB, shown as red ovals) 6

Influenza Nomenclature Specific varieties of the virus are generally named according to the particular antigenic determinants of hemagglutinin (15 major types) and neuraminidase (9 major types) surface proteins they possess, e. g. 7

8

Antigenic drift l l l Mutations in the antigenic structure of the influenza virus have resulted in a number of different influenza subtypes and strains. New strains of the influenza virus emerge due to a gradual process known as antigenic drift, in which mutations within the virus antibody-binding sites accumulate over time. Through this mechanism, the virus is able to largely circumvent the body's immune system, which may not be able to recognize and confer immunity to a new influenza strain even if an individual has already built up immunity to a different strain of the virus. Both A and B influenza viruses continually undergo antigenic drift, but the reformulation of influenza vaccines each year often enables scientists to take into account any new strains that have emerged. 9

10

Antigenic shift l l Influenza A also experiences another type of mutation called antigenic shift that results in a new subtype of the virus. Antigenic shift is a sudden change in antigenicity caused by the recombination of the influenza genome, which can occur when a cell becomes simultaneously infected by two different strains of type A influenza. The unusually broad range of hosts susceptible to influenza A appears to increase the likelihood that this event will occur. In particular, the mixing of strains that can infect birds, pigs, and humans is thought to be responsible for most antigenic shifts. Notably, in some parts of the world, humans live in close proximity to both swine and fowl, so that human strains and bird strains, may readily infect a pig at the same time, resulting in a unique virus. l New subtypes of influenza A develop abruptly and unpredictably so that scientists are unable to prepare vaccines in advance that are effective against them. Consequently, the emergence of a new subtype of the virus can cause a global pandemic in a very short amount of time. 11

Types of conventional trivalent influenza vaccines LAIV Live-attenuated influenza vaccines TIV Inactivated trivalent influenza vaccines CAIV-T Cold-adapted influenza vaccines trivalent -Whole virus vaccine -Split vaccine -Subunit vaccine -Virosome vaccine 12

13

LAIV - I l The LAIV vaccine consits of a master attenuated virus into which the HA and NA genes have been inserted. The master viruses for US vaccine: - A/Ann Arbor/6/60 (H 2 N 2) - B/Ann Arbor/1/66 The vaccine master virus is cold adapted – in other vords, it has been adapted to grow ideally at 25 degrees Celsius, which means that at normal human body temperature, it is attenuated. The adaptation process has been shown to have caused stable mutations in the three polymerase genes of the virus. (PA, PB 1 and PB 2) l l - The master viruses former SU vaccine: A/Leningrad/134/57 (H 2 N 2) ? ? ? B/USSR/60/69 ? ? ? 14

LAIV – II. l l Cold-adapted live attenuated influenza virus vaccines, for intranasal administration, have been available in the USA since July 2003. In the former Soviet Union live attenuated influenza vaccines have been used for several years. … The advatages of a live virus vaccine (for who likes it) -the application to the nasal mucosa and -the development of local neutralising immunity and -cell-mediated immune response. l Concerns: - the use in immunocompromised patients -the damage to mucosal surface (susceptibility to secondary infections) -the possibility of genetic reversion (change back to wild-type state) -the possibility of reassortment with wild-type influenza viruses (resulting in a new strain) l 15

TIV – I. (Killed vaccines) The backbone genes for killed vaccines are originated from the strain called PR 8. The master virus is A/Puerto Rico/8/34 (H 1 N 1) This strain is so attenuated that it is apathogenic and unable to replicate in humans. l l l Main steps of vaccine production: Influenza viruses are grown in the allantoic sac of embryonated hens’ eggs (or in cell culture) Subsequently purified and concentrated (using density gradient centrifugation or filter-membrane purification) Finally inactivated using formaldehid or β-propiolacton Whole virus vaccines were the first to be developed. Split vaccines are produced in the same way as whole virus vaccines, but virus particles are disrupted using detergents. Subunit vaccines consists of purified HA and NA proteins, the other viral components are removed. 16 of In virosome vaccines, the purified HA and NA are on the surface phospholipid virus like particles.

17

Whole virus vaccines I. l l Whole virus vaccines have the longest tradition. The firstly developed traditional whole virus vaccines demonstrated favourable immunogenicity results but before the last year experiences on H 1 N 1 mass vaccination, it was a widely accepted consensus that whole virus vaccines have a comparatively high reactogenicity. This is the explanation that whole virus seasonal flu vaccines are NOT licensed for use in children and have also been widely replaced in other age groups. The recent experiences are put question mark after this consensus! It seems not so sure! 18

Whole virus vaccines Fun in Hungary l l l In Hungary we have a national whole virus vaccine – and it is NOT recommended under the age of 3 years. But H 1 N 1 pandemic produced some exceptions – even is Hungary – the pandemic flu vaccine is applicable up to 1 year of age. So – we have a seasonal flu vaccine NOT applicable under 3 years of age – and a pandemic vaccine (produced in the same way, containing the same adjuvant, whole virus too) freely administered up to 1 year of age, as the lower age limit. 19

Who give the signal for seasonal flu vaccine production? What is the scientific basis of this signal? l l l The selection of virus strains is based on WHO recommendation. The scientific bases of this recommendation is the results which are coming from the WHO Global Influenza Surveillance System (GISS) - it means more than 100 laboratories throughout the world – constantly screen circulating influenza viruses for their antigenic constitution. Usually in February each year, the WHO recommend which three influenza strains should be used to formulate the vaccines for the coming season in the northern hemisphere. 20

Who give the virus for seasonal flu vaccine production? l l l „Referencec strains” = „vaccine strains” = „high -grow reassortants” are different names for the same. Influenza „A” viruses often grow poorly during manufacture. Industry funds the New York Medical College (NYMC) in the US, and the UK’s WHO Reference Laboratory (NIBSC) in the EU, to produce „high-grow” strains. (The vaccine manufacturer CSL in Australia and the LAIV vaccine producer Med. Immun in US are cover its own costs. ) The „high-grow” strains produced by NYMC and NIBSC are freely available to all manufacturer. 21

How long time have the producers? l l Producers have only a few month time for culture of the viruses, for antigen purification, for toxicity and immunogenicity analyses, for mass production and finally for the distribution of the new vaccine. So they have a rather strict time-line, and also for the regulators. The batch release of these vaccines, and the so called „yearly-licence” (small clinical trial) is a busy business for the regulators too. 22

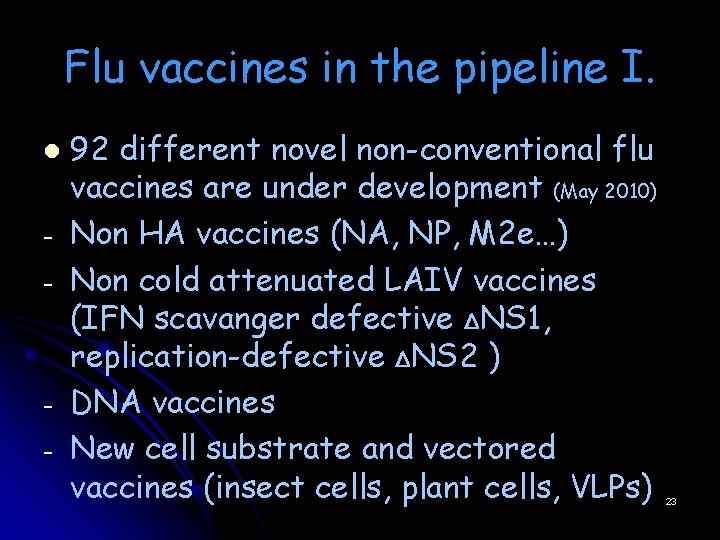
Flu vaccines in the pipeline I. l - - 92 different novel non-conventional flu vaccines are under development (May 2010) Non HA vaccines (NA, NP, M 2 e…) Non cold attenuated LAIV vaccines (IFN scavanger defective ΔNS 1, replication-defective ΔNS 2 ) DNA vaccines New cell substrate and vectored vaccines (insect cells, plant cells, VLPs) 23

24

l l l Flu vaccines in the pipeline II. DNA vaccines have been tested for a variety of viral and bacterial pathogens. The principle upon which the vaccine works is inoculation with DNA, which is taken up by antigen presenting cells, allowing them to produce viral proteins in their cytosol. These are then detected by the immune system, resulting in both a humoral and cellular immune response. Vaccines to conserved proteins have been considered, and among the candidates are the M 2 and the NP proteins. It is hoped that, by producing immunity to conserved proteins, i. e. proteins that do not undergo antigenic change like HA and NA do, a vaccine can be produced that does not need to be. reinvented. each year. This is also on the WHO. s agenda for a pandemic vaccine. Such vaccines have been shown to be effective in laboratory animals, but data are not available for human studies. Generic HA-based vaccines, aimed at conserved areas in the 25 protein, are also being considered.

Flu vaccines in the pipeline III. l l Attenuation by deletion of the gene NS 1 or decreasing the activity of NS 1 is being investigated. NS 1 produces a protein that inhibits the function of interferon alpha (IFNα). If a wild-type influenza virus infects a person, the NS 1 protein antagonises IFNα, which has an antiviral effect. An infection with a NS 1 -deficient virus would quickly be overcome by the immune system, hopefully resulting in an immune response, but with no symptoms. Replication-defective influenza viruses can be made by deleting the M 2 or the NS 2 genes (Hilleman 2002, Palese 2002). Only a single round of replication can occur, with termination before the formation of infectious viral particles. Protein expression will result in an immune response, and there is no danger 26 of infection spreading to other cells or people.

Reverz genetic Reverse genetics allows for specific manipulation of the influenza genome, exchanging genome segments for those desired. Based on this method, several plasmid-based methods (Neumann 2005) for constructing new viruses for vaccines have been developed. A number of plasmids, small circular pieces of DNA, containing the genes and promoter regions of the influenza virus, are transfected into cells, which are then capable of producing the viral genome segments and proteins to form a new viral particle. It may simplify and speed up the development of new vaccines, instead of the time consuming task of producing reassortment in eggs, and then searching for the correct reassortment (6 genes from the vaccine master strain, and HA and NA from the selected strain for the new vaccine), the vaccine producers could simply insert the HA and NA genes into a plasmid. 27

28
 Influenza virus replication
Influenza virus replication Influenza
Influenza Fibertel
Fibertel Piano di divisione delle staffe
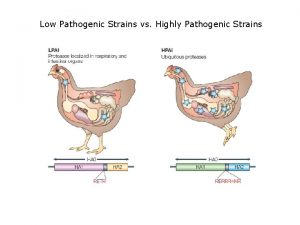
Piano di divisione delle staffe Low pathogenic avian influenza
Low pathogenic avian influenza Influenza
Influenza Influenza vaccine dosage chart 2019-2020
Influenza vaccine dosage chart 2019-2020 Causative agent
Causative agent Stomach flu vs influenza
Stomach flu vs influenza Influenza ww1
Influenza ww1 The great influenza rhetorical analysis essay
The great influenza rhetorical analysis essay Is influenza a airborne disease
Is influenza a airborne disease Rhinopneumonitis definition
Rhinopneumonitis definition Vaccins à vecteur viral
Vaccins à vecteur viral Sample rejection criteria
Sample rejection criteria Inklüzyon cisimcikleri
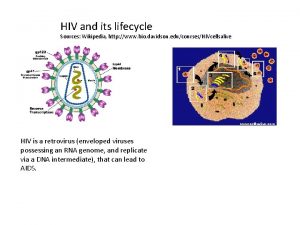
Inklüzyon cisimcikleri Hiv wikipedia
Hiv wikipedia Viral shedding
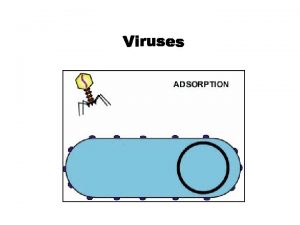
Viral shedding Section 24-1 viral structure and replication
Section 24-1 viral structure and replication The dynamics of viral marketing
The dynamics of viral marketing Viral infection
Viral infection Spasmodic croup
Spasmodic croup An acute highly contagious viral disease
An acute highly contagious viral disease Viral dna
Viral dna Meningitis
Meningitis Egg inoculation diagram
Egg inoculation diagram Vacinas subcutânea
Vacinas subcutânea Eline's viral
Eline's viral Hgado
Hgado