Chapter 18 Eukaryotic genomes fungi Jonathan Pevsner Ph



![Duplication of the S. cerevisiae genome Model [1] is favored for several reasons: -- Duplication of the S. cerevisiae genome Model [1] is favored for several reasons: --](https://slidetodoc.com/presentation_image/2f3169bff2e548cc3e40ea03140c9c97/image-50.jpg)




- Slides: 84

Chapter 18: Eukaryotic genomes: fungi Jonathan Pevsner, Ph. D. pevsner@kennedykrieger. org Bioinformatics and Functional Genomics (Wiley-Liss, 3 rd edition, 2015) You may use this Power. Point for teaching

Learning objectives After reading this chapter you should be able to: ■ provide an overview of how fungi are classified; ■ describe the features of the Saccharomyces cerevisiae genome; ■ discuss genome duplication in S. cerevisiae; ■ describe comparative genomics of the genus Saccharomyces; and ■ describe comparative genomics of other fungal phyla.

Outline Introduction Description and classification of fungi Introduction to budding yeast Saccharomyces cerevisiae Gene duplication and genome duplication Comparative analyses of hemiascomycetes Analysis of fungal genomes Perspective

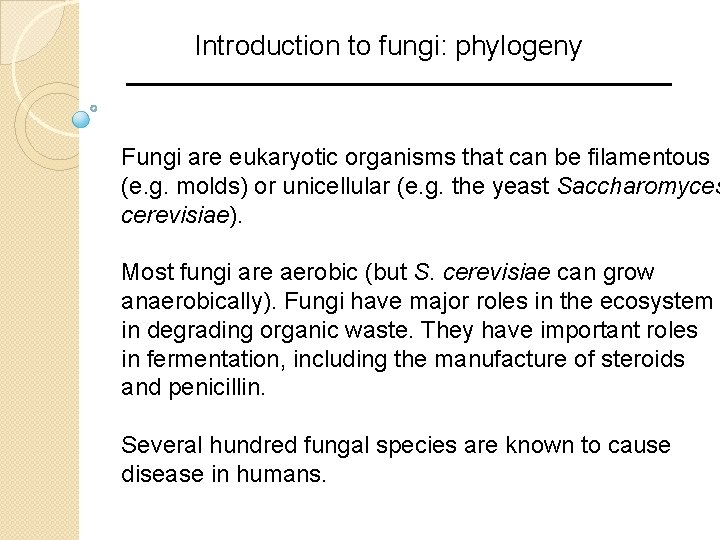
Introduction to fungi: phylogeny Fungi are eukaryotic organisms that can be filamentous (e. g. molds) or unicellular (e. g. the yeast Saccharomyces cerevisiae). Most fungi are aerobic (but S. cerevisiae can grow anaerobically). Fungi have major roles in the ecosystem in degrading organic waste. They have important roles in fermentation, including the manufacture of steroids and penicillin. Several hundred fungal species are known to cause disease in humans.

Fungi are a sister group with the metazoans (animals) B&FG 3 e Fig. 18. 1 Page 849 We return to this phylogenetic tree in Chapter 19

Outline Introduction Description and classification of fungi Introduction to budding yeast Saccharomyces cerevisiae Gene duplication and genome duplication Comparative analyses of hemiascomycetes Analysis of fungal genomes Perspective

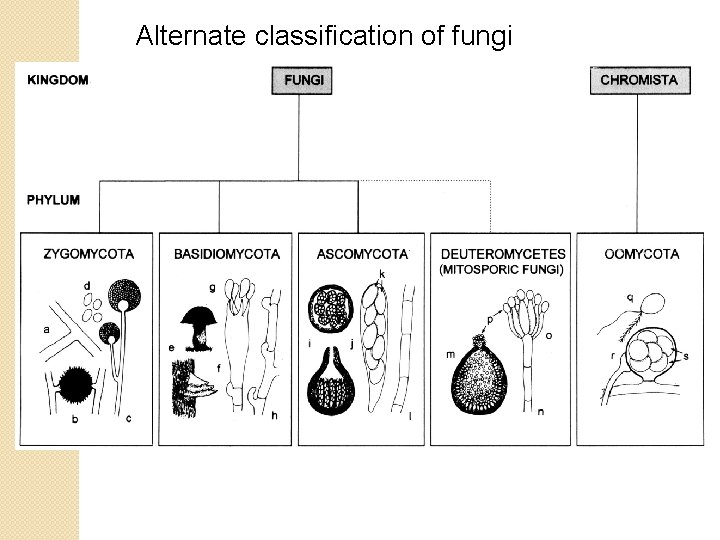
Classification of fungi About 70, 000 fungal species have been described (as of 1995), but 1. 5 million species may exist. Four phyla: Ascomycota Basidiomycota mushrooms Chytridiomycota Zygomycota vegetation yeasts, truffles, lichens rusts, smuts, Allomyces feed on decaying

Classification of fungi About 70, 000 fungal species have been described (as of 1995), but 1. 5 million species may exist. Four phyla: Ascomycota yeasts, truffles, lichens Hemiascomycetae Génolevure project Euascomycetae Neurospora Loculoascomycetae Laboulbeniomycetae parasites of insects Basidiomycota rusts, smuts, mushrooms Chytridiomycota Allomyces Zygomycota feed on decaying

Alternate classification of fungi

Outline Introduction Description and classification of fungi Introduction to budding yeast Saccharomyces cerevisiae Gene duplication and genome duplication Comparative analyses of hemiascomycetes Analysis of fungal genomes Perspective

Introduction to Saccharomyces cerevisiae First species domesticated by humans Called baker’s yeast (or brewer’s yeast) Ferments glucose to ethanol and carbon dioxide Model organism for studies of biochemistry, genetics, molecular and cell biology …rapid growth rate …easy to modify genetically …features typical of eukaryotes …relatively simple (unicellular) …relatively small genome

Sequencing the S. cerevisiae genome The genome was sequenced by a highly cooperative consortium in the early 1990 s, chromosome by chromosom (the whole genome shotgun approach was not used). This involved 600 researchers in > 100 laboratories. --Physical map created for all XVI chromosomes --Library of 10 kb inserts constructed in phage --The inserts were assembled into contigs The sequence released in 1996, and published in 1997 (Goffeau et al. , 1996; Mewes et al. , 1997)

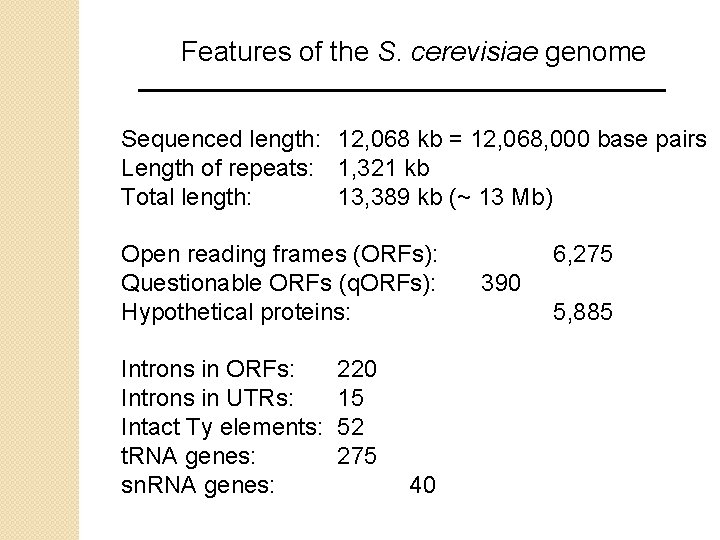
Features of the S. cerevisiae genome Sequenced length: 12, 068 kb = 12, 068, 000 base pairs Length of repeats: 1, 321 kb Total length: 13, 389 kb (~ 13 Mb) Open reading frames (ORFs): Questionable ORFs (q. ORFs): Hypothetical proteins: Introns in ORFs: Introns in UTRs: Intact Ty elements: t. RNA genes: sn. RNA genes: 220 15 52 275 40 6, 275 390 5, 885

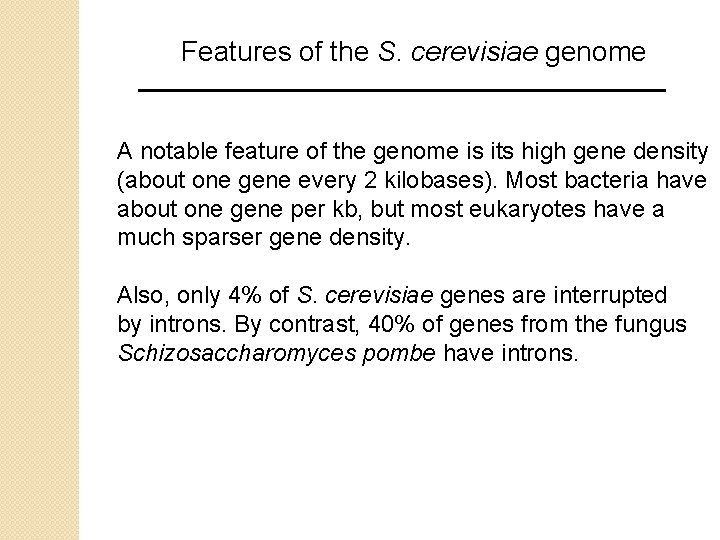
Features of the S. cerevisiae genome A notable feature of the genome is its high gene density (about one gene every 2 kilobases). Most bacteria have about one gene per kb, but most eukaryotes have a much sparser gene density. Also, only 4% of S. cerevisiae genes are interrupted by introns. By contrast, 40% of genes from the fungus Schizosaccharomyces pombe have introns.

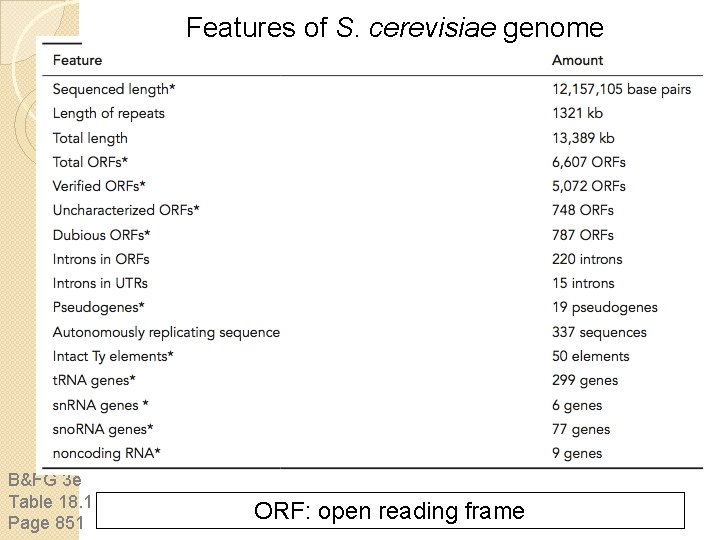
Features of S. cerevisiae genome B&FG 3 e Table 18. 1 Page 851 ORF: open reading frame

ORFs in the S. cerevisiae genome How are ORFs defined? In the initial genome analysis, an ORF was defined as >100 codons (thus specifying a protein of ~11 kilodaltons). 390 ORFs were listed as “questionable”, because they were considered unlikely to be authentic genes. For example, they were short, or exhibited unlikely preferences for codon usage. How many ORFs are there in the yeast genome? There are 40, 000 ORFs > 20 amino acids; how many of these are authentic?

ORFs in the S. cerevisiae genome Several criteria may be applied to decide if ORFs are authentic protein-coding genes: [1] evidence of conservation in other organisms [2] experimental evidence of gene expression (microarrays, SAGE, functional genomics) The groups of Elizabeth Winzeler and Michael Snyder each described hundreds of previously unannotated genes that are transcribed and translated.

Revising the S. cerevisiae gene count through comparative genomics By sequencing three additional yeast species (Saccharomyces paradoxus, S. bayanus, S. mikatae), Kellis et al. showed that 503 genes should be deleted from the set of yeast genes (leaving 5, 726 including 43 newly discovered genes). See Kellis et al. (2003) Nature 423: 241.

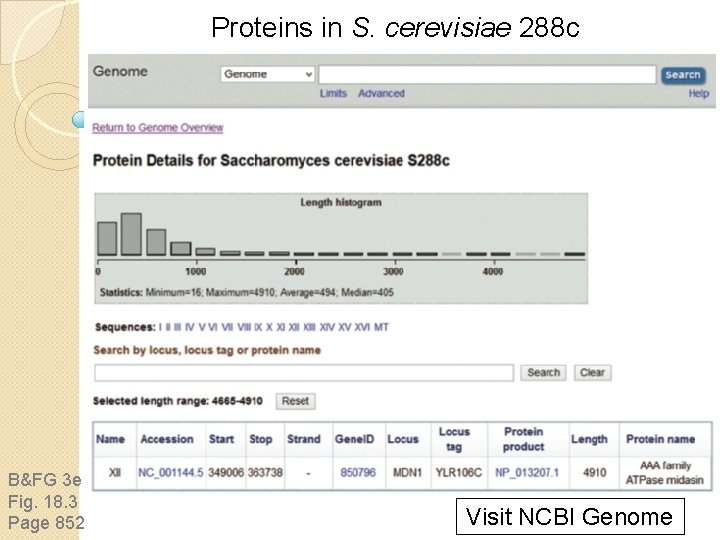
Proteins in S. cerevisiae 288 c B&FG 3 e Fig. 18. 3 Page 852 Visit NCBI Genome

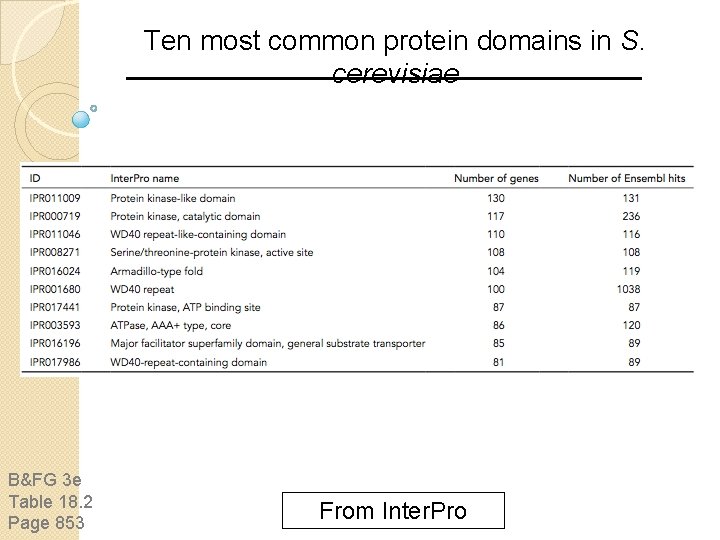
Ten most common protein domains in S. cerevisiae B&FG 3 e Table 18. 2 Page 853 From Inter. Pro

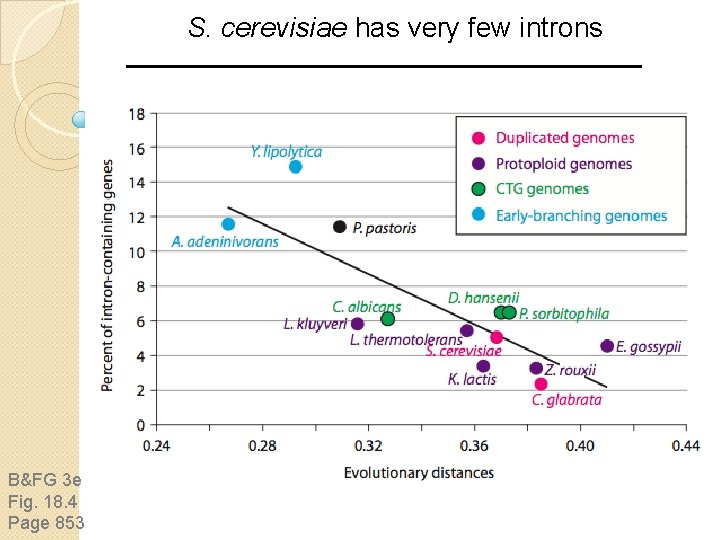
S. cerevisiae has very few introns B&FG 3 e Fig. 18. 4 Page 853

Exploring a typical S. cerevisiae chromosome (XII) We will next familiarize ourselves with the S. cerevisiae genome by exploring a typical chromosome, XII. This chromosome features • 38% GC content • very little repetitive DNA • few introns • six Ty elements (transposable elements) • a high ORF density: 534 ORFs > 100 aa, and 72% of the chromosome has protein-coding genes B&FG 3 e Page 854

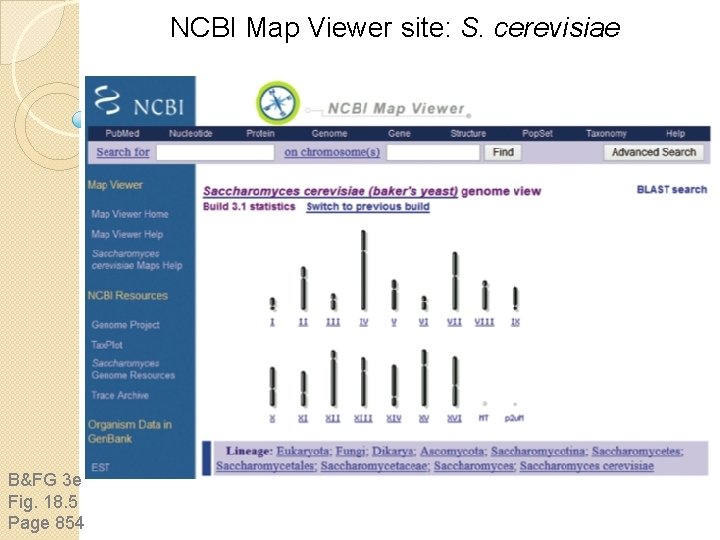
NCBI Map Viewer site: S. cerevisiae B&FG 3 e Fig. 18. 5 Page 854

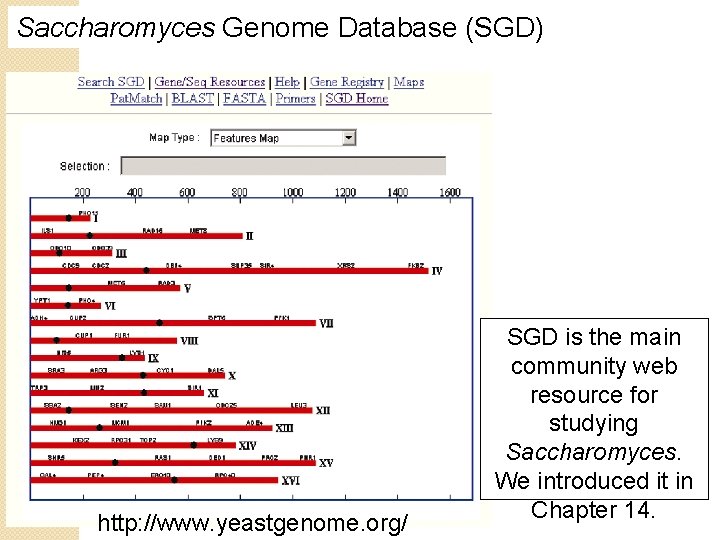
Saccharomyces Genome Database (SGD) http: //www. yeastgenome. org/ SGD is the main community web resource for studying Saccharomyces. We introduced it in Chapter 14.

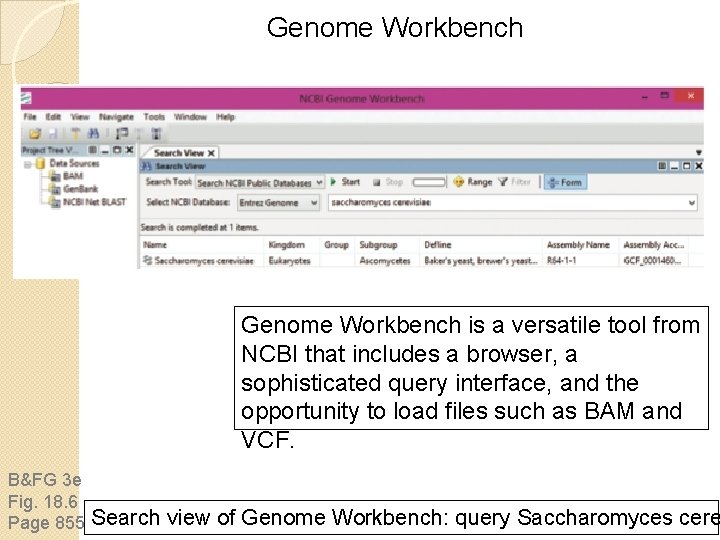
Genome Workbench is a versatile tool from NCBI that includes a browser, a sophisticated query interface, and the opportunity to load files such as BAM and VCF. B&FG 3 e Fig. 18. 6 Page 855 Search view of Genome Workbench: query Saccharomyces cere

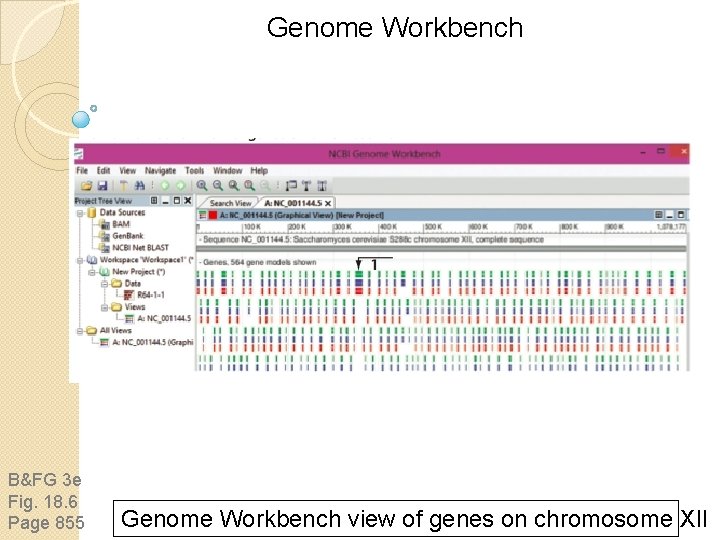
Genome Workbench B&FG 3 e Fig. 18. 6 Page 855 Genome Workbench view of genes on chromosome XII

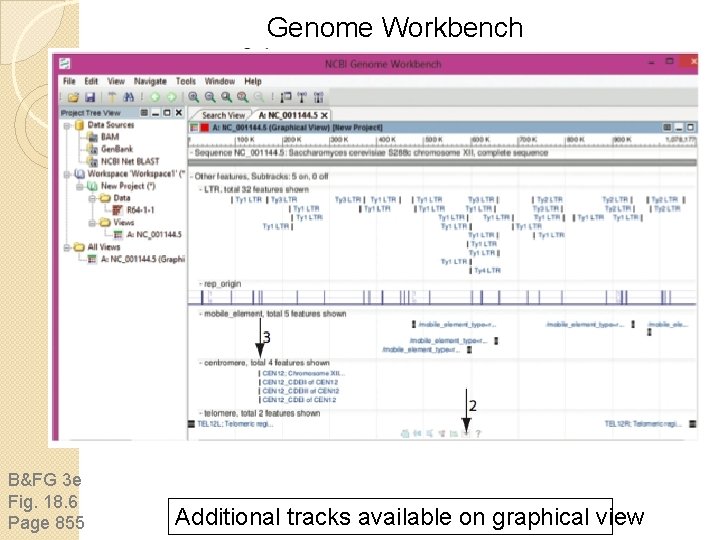
Genome Workbench B&FG 3 e Fig. 18. 6 Page 855 Additional tracks available on graphical view

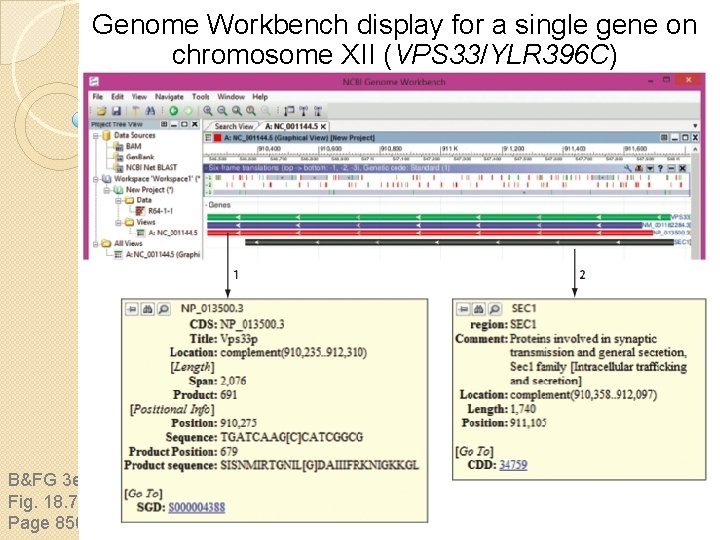
Genome Workbench display for a single gene on chromosome XII (VPS 33/YLR 396 C) B&FG 3 e Fig. 18. 7 Page 856

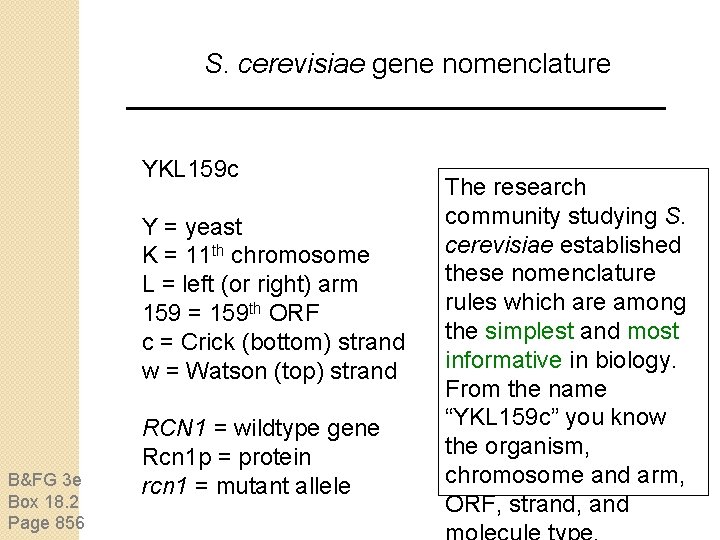
S. cerevisiae gene nomenclature YKL 159 c Y = yeast K = 11 th chromosome L = left (or right) arm 159 = 159 th ORF c = Crick (bottom) strand w = Watson (top) strand B&FG 3 e Box 18. 2 Page 856 RCN 1 = wildtype gene Rcn 1 p = protein rcn 1 = mutant allele The research community studying S. cerevisiae established these nomenclature rules which are among the simplest and most informative in biology. From the name “YKL 159 c” you know the organism, chromosome and arm, ORF, strand, and

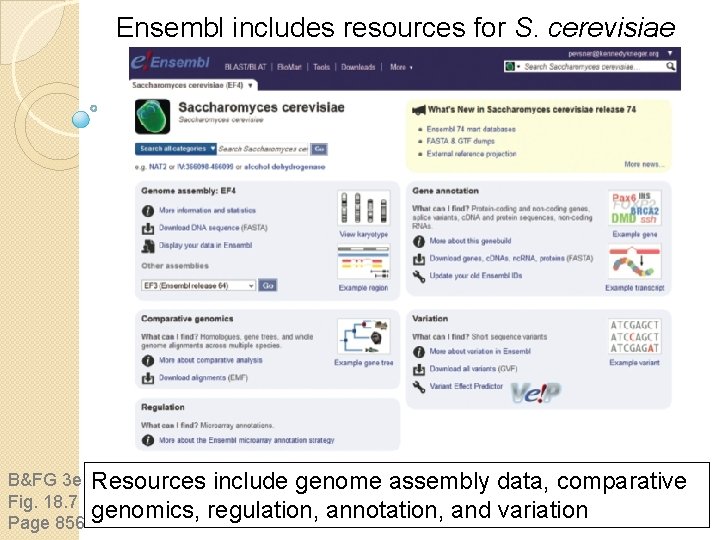
Ensembl includes resources for S. cerevisiae B&FG 3 e Fig. 18. 7 Page 856 Resources include genome assembly data, comparative genomics, regulation, annotation, and variation

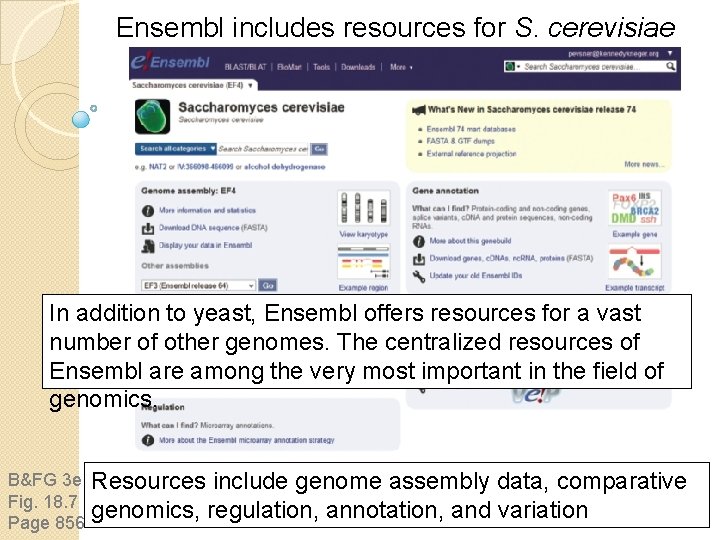
Ensembl includes resources for S. cerevisiae In addition to yeast, Ensembl offers resources for a vast number of other genomes. The centralized resources of Ensembl are among the very most important in the field of genomics. B&FG 3 e Fig. 18. 7 Page 856 Resources include genome assembly data, comparative genomics, regulation, annotation, and variation

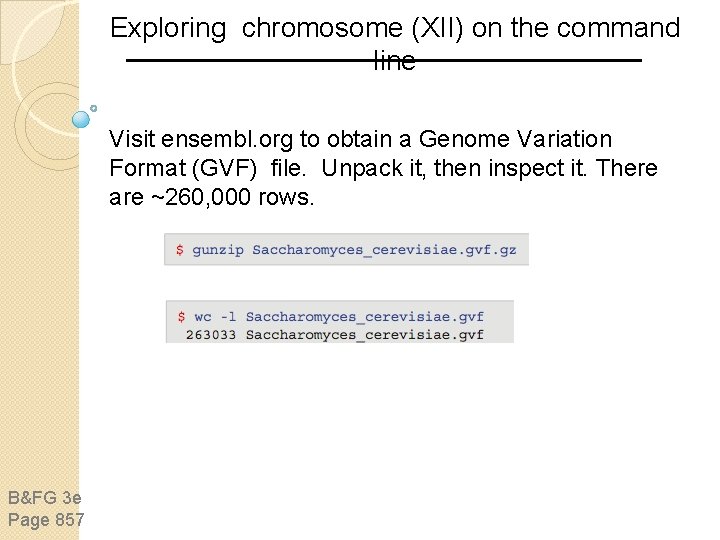
Exploring chromosome (XII) on the command line Visit ensembl. org to obtain a Genome Variation Format (GVF) file. Unpack it, then inspect it. There are ~260, 000 rows. B&FG 3 e Page 857

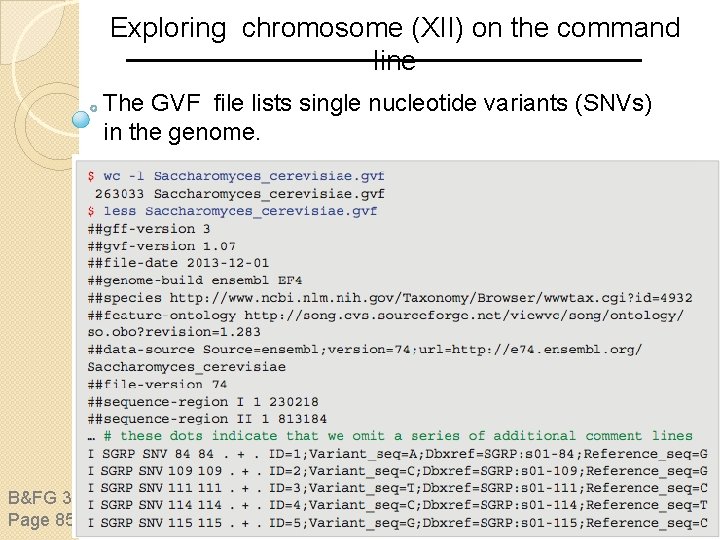
Exploring chromosome (XII) on the command line The GVF file lists single nucleotide variants (SNVs) in the genome. B&FG 3 e Page 858

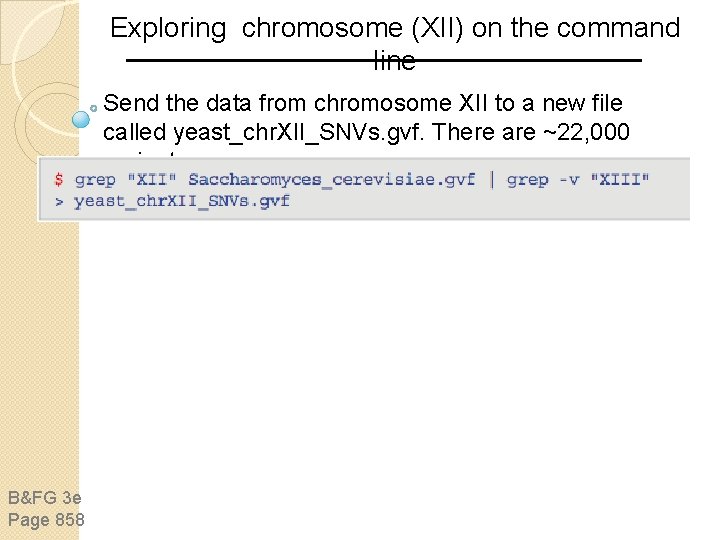
Exploring chromosome (XII) on the command line Send the data from chromosome XII to a new file called yeast_chr. XII_SNVs. gvf. There are ~22, 000 variants. B&FG 3 e Page 858

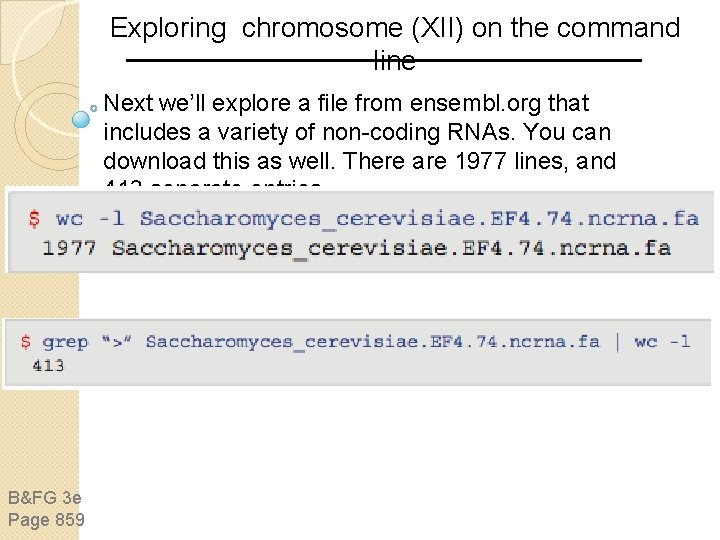
Exploring chromosome (XII) on the command line Next we’ll explore a file from ensembl. org that includes a variety of non-coding RNAs. You can download this as well. There are 1977 lines, and 413 separate entries. B&FG 3 e Page 859

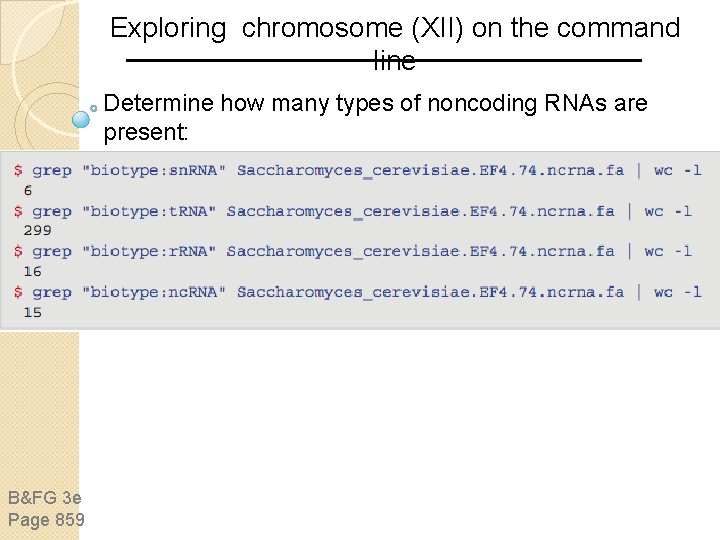
Exploring chromosome (XII) on the command line Determine how many types of noncoding RNAs are present: B&FG 3 e Page 859

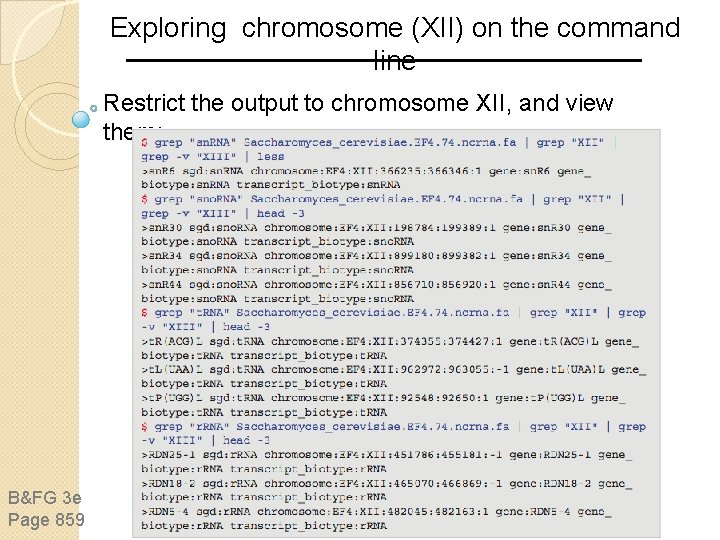
Exploring chromosome (XII) on the command line Restrict the output to chromosome XII, and view them: B&FG 3 e Page 859

Outline Introduction Description and classification of fungi Introduction to budding yeast Saccharomyces cerevisiae Gene duplication and genome duplication Comparative analyses of hemiascomycetes Analysis of fungal genomes Perspective

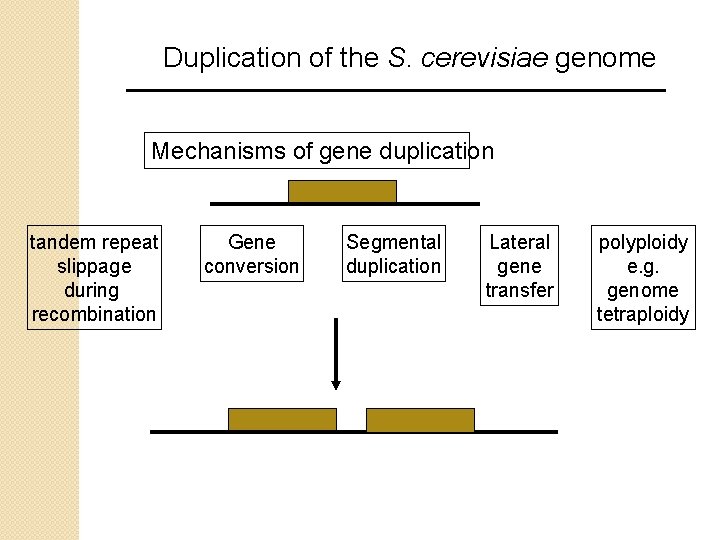
Duplication of the S. cerevisiae genome Analysis of the S. cerevisiae genome revealed that many regions are duplicated, both intrachromosomally and interchromosomally (within and between chromosomes). These duplicated regions include both genes and nongenic regions. Such duplications reflect a fundamental aspect of genome evolution. What are the mechanisms by which regions of the genome duplicate?

Duplication of the S. cerevisiae genome Mechanisms of gene duplication tandem repeat slippage during recombination Gene conversion Segmental duplication Lateral gene transfer polyploidy e. g. genome tetraploidy

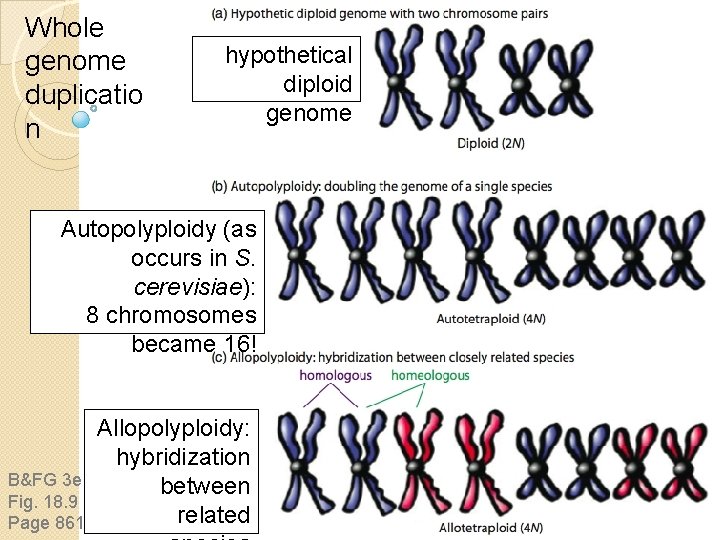
Whole genome duplicatio n hypothetical diploid genome Autopolyploidy (as occurs in S. cerevisiae): 8 chromosomes became 16! B&FG 3 e Fig. 18. 9 Page 861 Allopolyploidy: hybridization between related

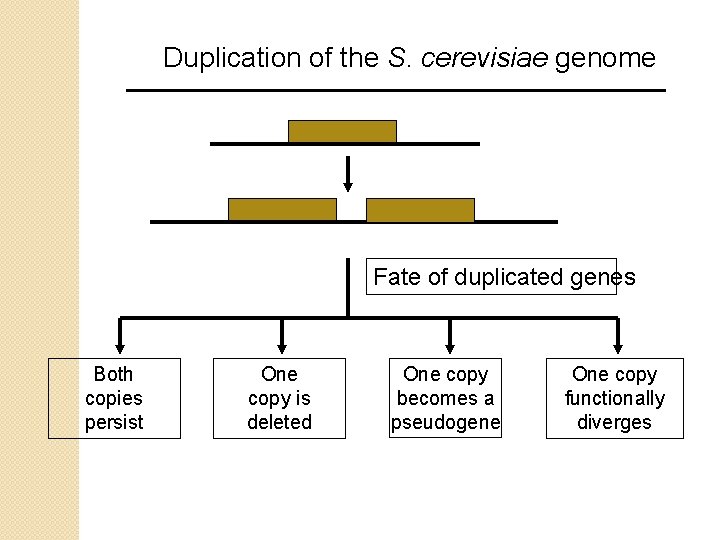
Duplication of the S. cerevisiae genome Fate of duplicated genes Both copies persist One copy is deleted One copy becomes a pseudogene One copy functionally diverges

Duplication of the S. cerevisiae genome What is the fate of duplicated genes? (see YGOB, below) A duplicated gene (overall in eukaryotes) has a half life of just several million years (Lynch and Conery, 2000). 50% to 92% of duplicated genes are lost (Wagner, 2001) Consider four possible fates of a duplicated gene: [1] Both copies persist (gene dosage effect) [2] One copy is deleted (a common fate) [3] One copy accumulates mutations and becomes a pseudogene (no functional protein product) [4] One copy (or both) diverges functionally. The organism can perform a novel function.

Duplication of the S. cerevisiae genome In 1970, Susumu Ohno published the book Evolution by Gene Duplication. He hypothesized that vertebrate genomes evolved by two rounds of whole genome duplication. This provided genomes with the “raw materials” (new genes) with which to introduce various innovations.

Duplication of the S. cerevisiae genome Ohno (1970): “Had evolution been entirely dependent upon natural selection, from a bacterium only numerous forms of bacteria would have emerged. The creation of metazoan vertebrates, and finally mammals from unicellular organisms would have been quite impossible, for such big leaps in evolution required the creation of new gene loci with previously nonexistent function. Only the cistron that became redundant was able to escape from the relentless pressure of natural selection. By escaping, it accumulated formerly forbidden mutations to emerge as a new gene locus. ”

Duplication of the S. cerevisiae genome Wolfe and Shields (1997, Nature) provided support for Ohno’s paradigm. They hypothesized that the yeast genome duplicated about 100 million years ago. There was a diploid yeast genome with about 5, 000 genes. It doubled to a tetraploid number of 10, 000 genes. Then there was massive gene loss and chromosomal rearrangement to yield the present day 6, 000 genes.

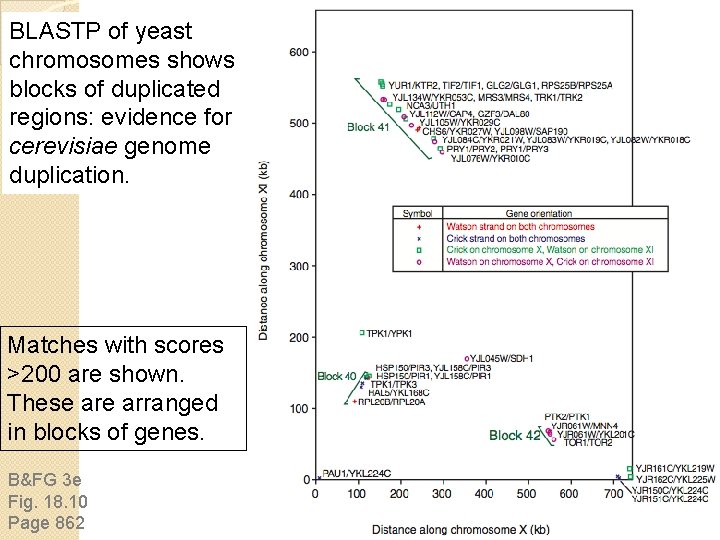
BLASTP of yeast chromosomes shows 55 blocks of duplicated regions: evidence for S. cerevisiae genome duplication. Matches with scores >200 are shown. These arranged in blocks of genes. B&FG 3 e Fig. 18. 10 Page 862

Duplication of the S. cerevisiae genome Evidence of genome duplication in yeast -- Systematic BLAST searches show 55 blocks of duplicated sequences. -- There are 376 pairs of homologous genes. You can see the results of chromosomal comparisons on Ken Wolfe’s web site (YGOG; see below for examples) and at the SGD web site.

Duplication of the S. cerevisiae genome Two models for the presence of duplication blocks [1] Whole genome duplication (tetraploidy) followed by gene loss and rearrangements [2] Successive, independent duplication events
![Duplication of the S cerevisiae genome Model 1 is favored for several reasons Duplication of the S. cerevisiae genome Model [1] is favored for several reasons: --](https://slidetodoc.com/presentation_image/2f3169bff2e548cc3e40ea03140c9c97/image-50.jpg)
Duplication of the S. cerevisiae genome Model [1] is favored for several reasons: -- For 50 of 55 duplicated regions, the orientation of the entire block is preserved with respect to the centromere. The orientation is not random. -- For model [2] we would expect 7 triplicated regions. We observe only 0 or 1. -- Gene order is maintained in 14 hemiascomycetes (the Génolevures project) Page 711

Duplication of the S. cerevisiae genome Why are duplicated genes commonly lost? It might seem highly advantageous to have a second copy of gene, thus permitting functional divergence. Ohno suggested two reasons: [1] After duplication, a deleterious mutation in one of the two genes might now persist. Without duplication, the individual would have been selected against by such a mutation. [2] The presence of a new paralogous sequence could lead to unequal crossing over of homologous

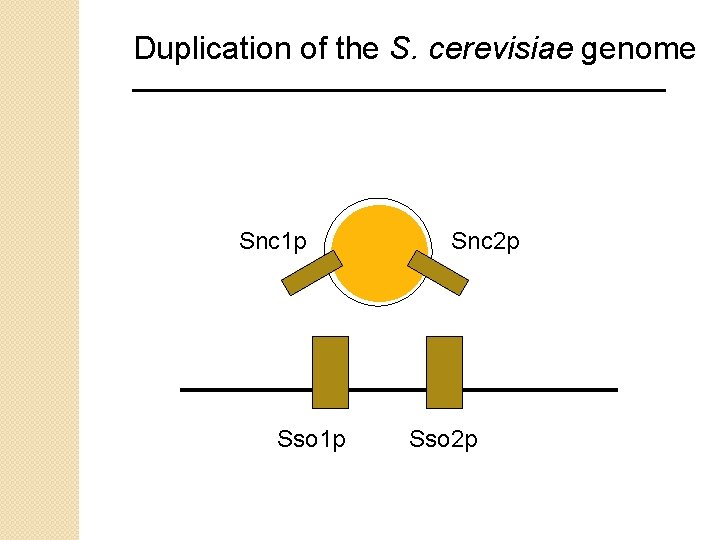
Duplication of the S. cerevisiae genome To consider the fate of duplicated genes, consider the example of genes involved in vesicle transport. Vesicles carry cargo from one destination to another. Proteins on vesicles (e. g. vesicle-associated membrane protein, VAMP; Snc 1 p in yeast) bind to proteins on target membranes (e. g. syntaxin in mammalian and other eukaryotic systems, or Sso 1 p in yeast). In S. cerevisiae, genome duplication appears to be responsible for the presence of two syntaxins

Duplication of the S. cerevisiae genome Snc 1 p Sso 1 p Snc 2 p Sso 2 p

Search for information on SSO 1 (or any yeast gene) at the SGD website

The SGD record for SSO 1 provides information on functio

Duplication of the S. cerevisiae genome The SGD website reveals that the SSO 1 gene is nonessential (i. e. the null mutant is viable), but the double knockout of SSO 1 and SSO 1 is lethal. Thus, these paralogs may offer functional redundancy to the organism. Also, these proteins could participate in distinct (but complementary) intracellular trafficking steps.

Outline Introduction Description and classification of fungi Introduction to budding yeast Saccharomyces cerevisiae Gene duplication and genome duplication Comparative analyses of hemiascomycetes Analysis of fungal genomes Perspective

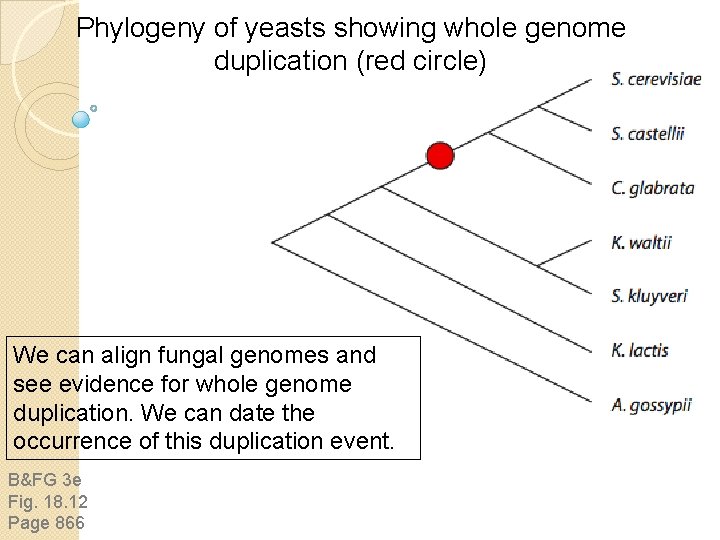
Phylogeny of yeasts showing whole genome duplication (red circle) We can align fungal genomes and see evidence for whole genome duplication. We can date the occurrence of this duplication event. B&FG 3 e Fig. 18. 12 Page 866

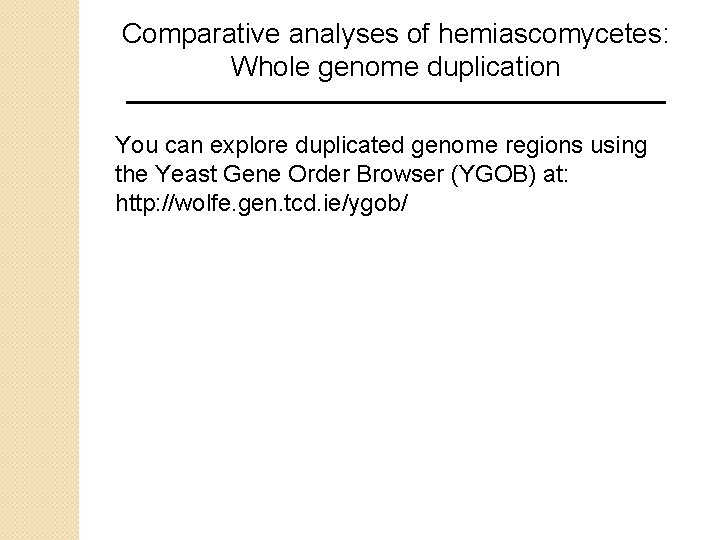
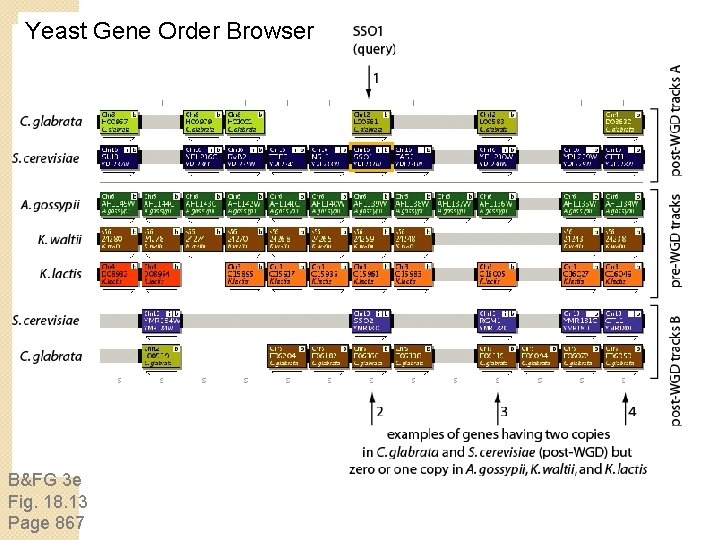
Comparative analyses of hemiascomycetes: Whole genome duplication You can explore duplicated genome regions using the Yeast Gene Order Browser (YGOB) at: http: //wolfe. gen. tcd. ie/ygob/

Yeast Gene Order Browser B&FG 3 e Fig. 18. 13 Page 867

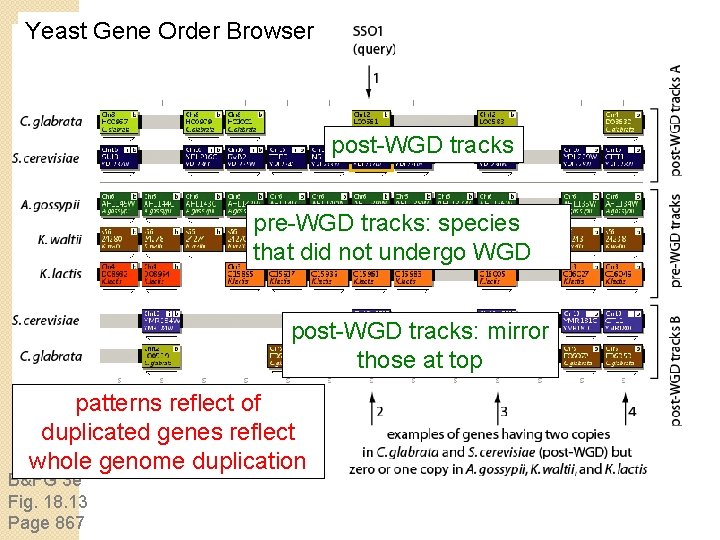
Yeast Gene Order Browser post-WGD tracks pre-WGD tracks: species that did not undergo WGD post-WGD tracks: mirror those at top patterns reflect of duplicated genes reflect whole genome duplication B&FG 3 e Fig. 18. 13 Page 867

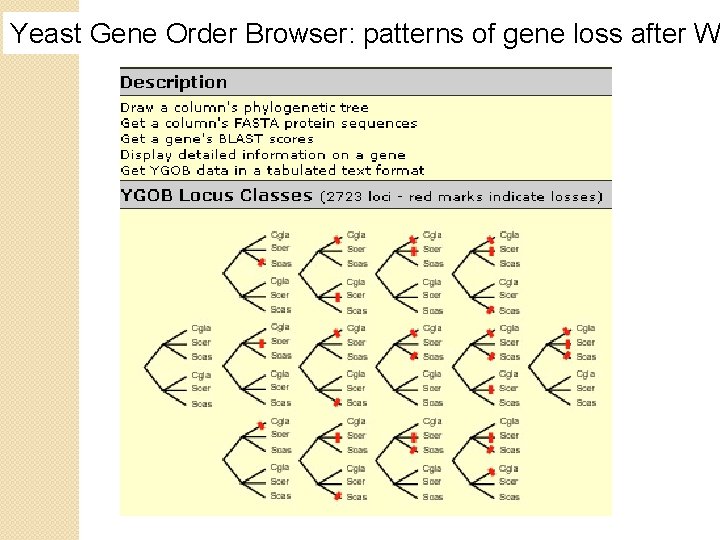
Yeast Gene Order Browser: patterns of gene loss after W

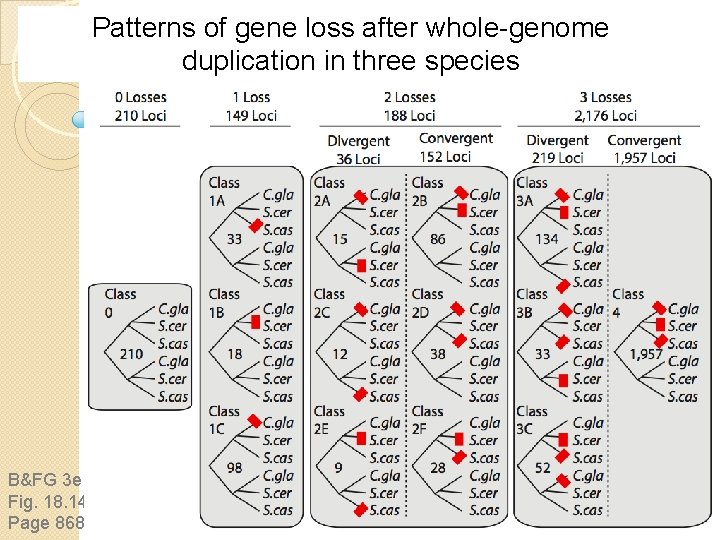
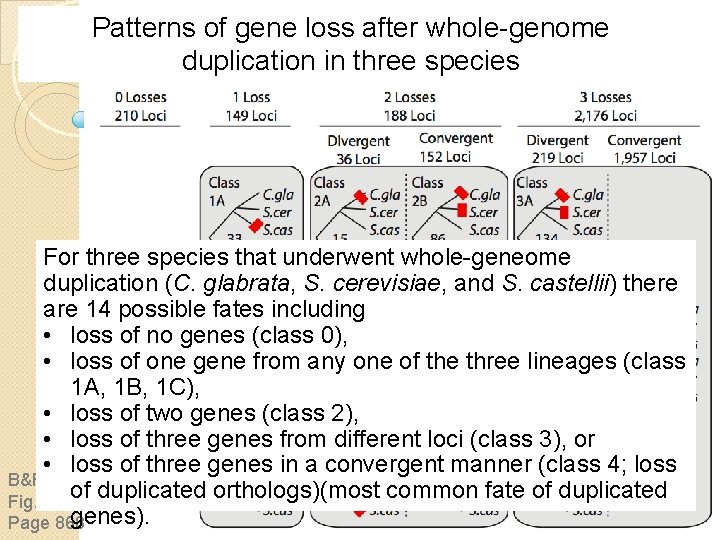
Patterns of gene loss after whole-genome duplication in three species B&FG 3 e Fig. 18. 14 Page 868

Patterns of gene loss after whole-genome duplication in three species For three species that underwent whole-geneome duplication (C. glabrata, S. cerevisiae, and S. castellii) there are 14 possible fates including • loss of no genes (class 0), • loss of one gene from any one of the three lineages (class 1 A, 1 B, 1 C), • loss of two genes (class 2), • loss of three genes from different loci (class 3), or • loss of three genes in a convergent manner (class 4; loss B&FG 3 e of duplicated orthologs)(most common fate of duplicated Fig. 18. 14 genes). Page 868

Comparative analyses of hemiascomycetes: Identification of functional elements Kellis et al. (2003) compared S. paradoxus, S. mikatae, and S. bayanus to S. cerevisiae (divergence dates: 5 to 20 MYA). There were clear orthologous matches, except at the telomeres. For the Gal 4 transcription factor and other functional elements, comparative analyses have helped delineate regulatory regions.

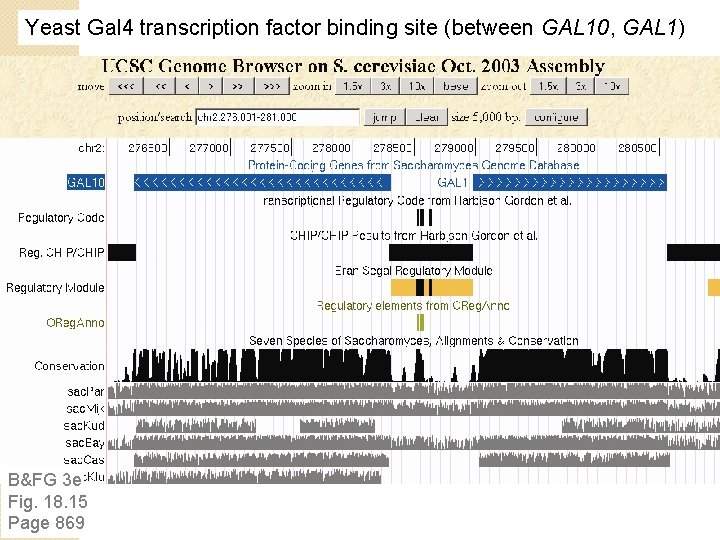
Yeast Gal 4 transcription factor binding site (between GAL 10, GAL 1) B&FG 3 e Fig. 18. 15 Page 869

Outline Introduction Description and classification of fungi Introduction to budding yeast Saccharomyces cerevisiae Gene duplication and genome duplication Comparative analyses of hemiascomycetes Analysis of fungal genomes Perspective

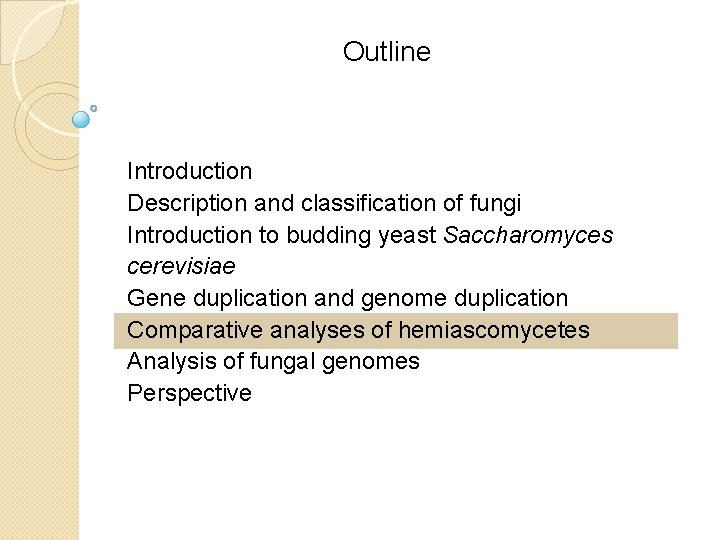
Fungal genome projects: representative examples of the Ascomycetes B&FG 3 e Table 18. 3 Page 869 ID refers to the NCBI genome project identifier

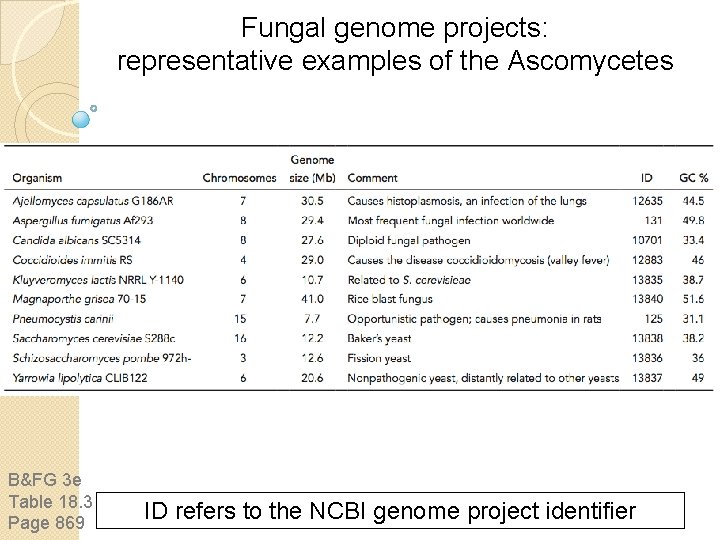
Fungal genome projects: representative examples of the Basidiomycetes B&FG 3 e Table 18. 3 Page 869 ID refers to the NCBI genome project identifier

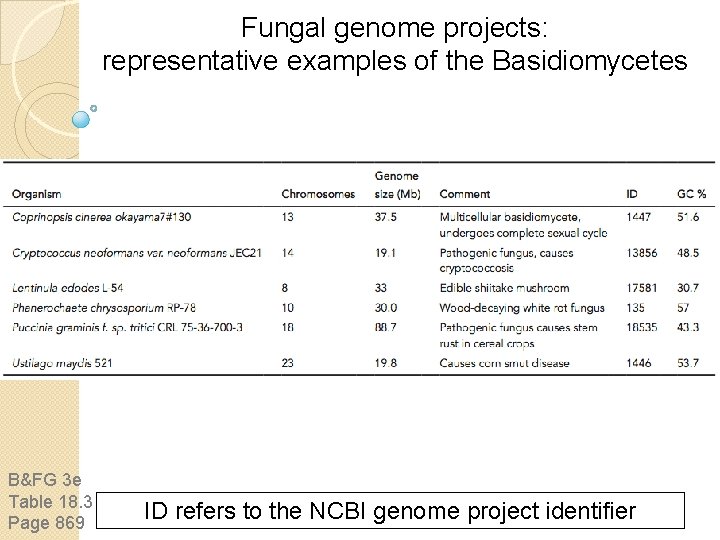
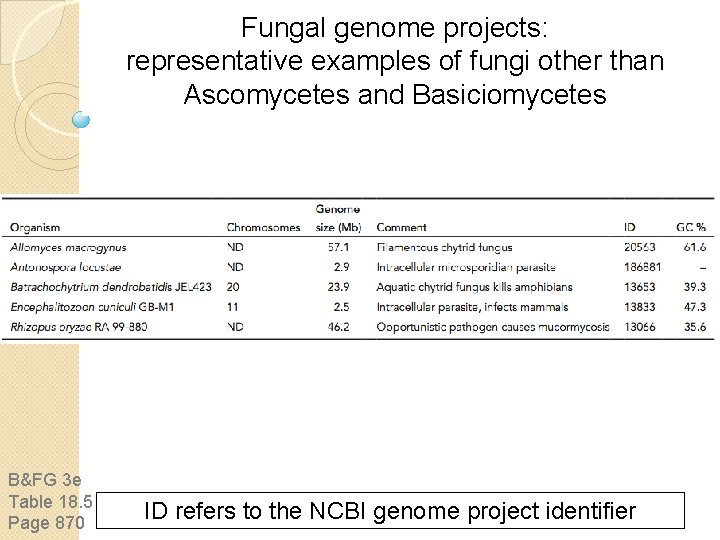
Fungal genome projects: representative examples of fungi other than Ascomycetes and Basiciomycetes B&FG 3 e Table 18. 5 Page 870 ID refers to the NCBI genome project identifier

Fungal genomes We now briefly consider a set of prominent fungal genomes, arranged alphabetically: Aspergillus nidulans Candida albicans Cryptococcus neoformans: model fungal pathogen Encephalitozoon cuniculi: atypical fungus Neurospora crassa Phanerochaete chrysosporium: first Basidiomycete Schizosaccharomyces pombe: fission yeast

Fungal pathogen: Aspergillus nidulans --Of 185 Aspergillus species, 20 are human pathogens --A. nidulans has a sexual life cycle (in contrast to A. fumigatus and A. oryzae [sake, miso, soy]). --A. nidulans has animal-like peroxisomal enzymes

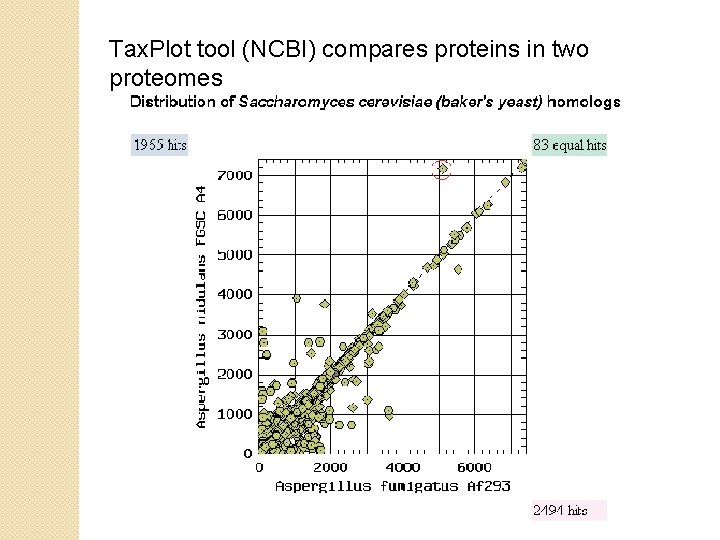
Tax. Plot tool (NCBI) compares proteins in two proteomes

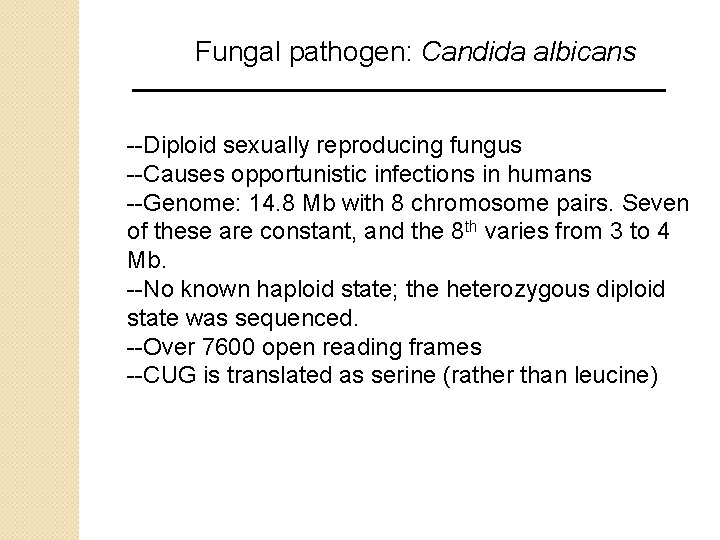
Fungal pathogen: Candida albicans --Diploid sexually reproducing fungus --Causes opportunistic infections in humans --Genome: 14. 8 Mb with 8 chromosome pairs. Seven of these are constant, and the 8 th varies from 3 to 4 Mb. --No known haploid state; the heterozygous diploid state was sequenced. --Over 7600 open reading frames --CUG is translated as serine (rather than leucine)

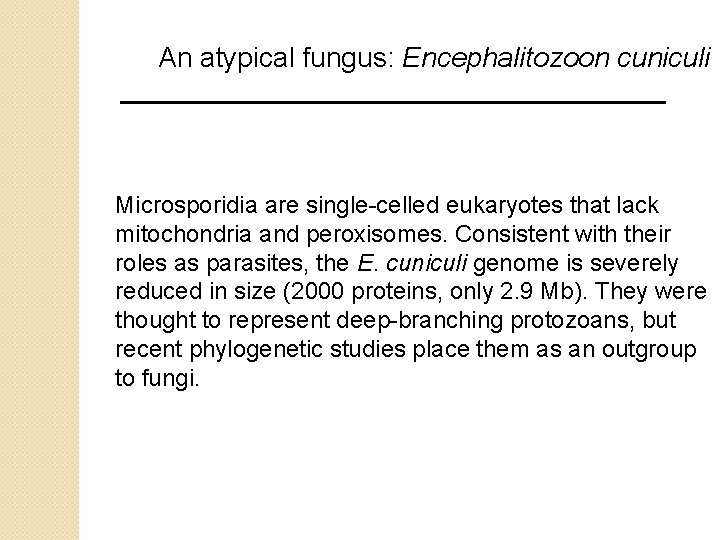
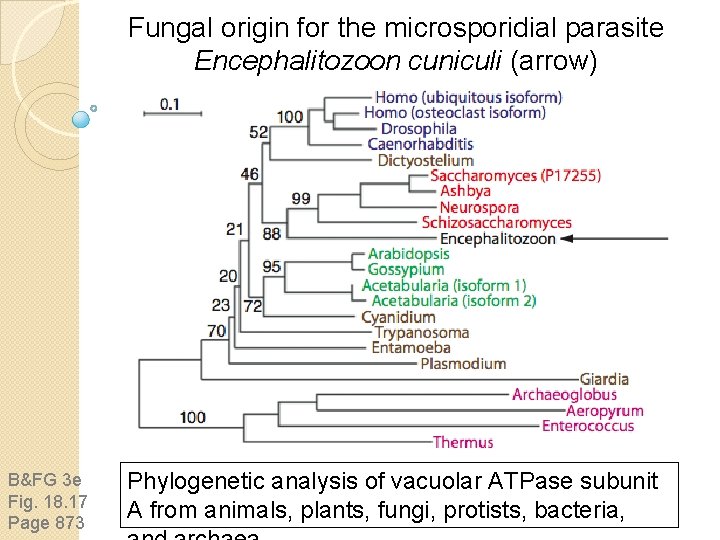
An atypical fungus: Encephalitozoon cuniculi Microsporidia are single-celled eukaryotes that lack mitochondria and peroxisomes. Consistent with their roles as parasites, the E. cuniculi genome is severely reduced in size (2000 proteins, only 2. 9 Mb). They were thought to represent deep-branching protozoans, but recent phylogenetic studies place them as an outgroup to fungi.

Fungal origin for the microsporidial parasite Encephalitozoon cuniculi (arrow) B&FG 3 e Fig. 18. 17 Page 873 Phylogenetic analysis of vacuolar ATPase subunit A from animals, plants, fungi, protists, bacteria,

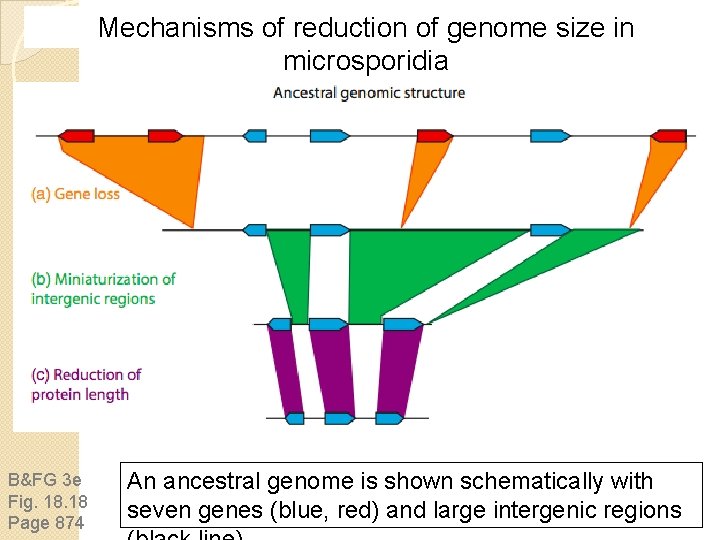
Mechanisms of reduction of genome size in microsporidia B&FG 3 e Fig. 18 Page 874 An ancestral genome is shown schematically with seven genes (blue, red) and large intergenic regions

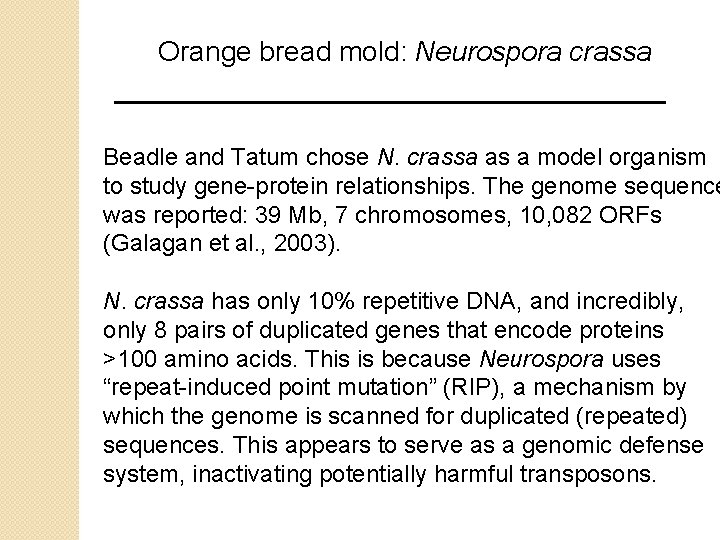
Orange bread mold: Neurospora crassa Beadle and Tatum chose N. crassa as a model organism to study gene-protein relationships. The genome sequence was reported: 39 Mb, 7 chromosomes, 10, 082 ORFs (Galagan et al. , 2003). N. crassa has only 10% repetitive DNA, and incredibly, only 8 pairs of duplicated genes that encode proteins >100 amino acids. This is because Neurospora uses “repeat-induced point mutation” (RIP), a mechanism by which the genome is scanned for duplicated (repeated) sequences. This appears to serve as a genomic defense system, inactivating potentially harmful transposons.

Phanerochaete chrysosporium: first Basidiomyce • Basidiomycota diverged from the bettercharacterized Ascomycota over 500 million years ago. • Genome: 30 Mb of DNA, 10 chromosomes. • 11, 777 genes predicted. • White rot fungi (such as P. chrysosporium) degrade the major components of plant cell walls, including cellulose and lignins, using a series of oxidases and peroxidases. The genome encodes hundreds of enzymes that are able to cleave carbohydrates. • Comparative analyses of 31 fungal genomes suggests the emergence of white rot with wood-

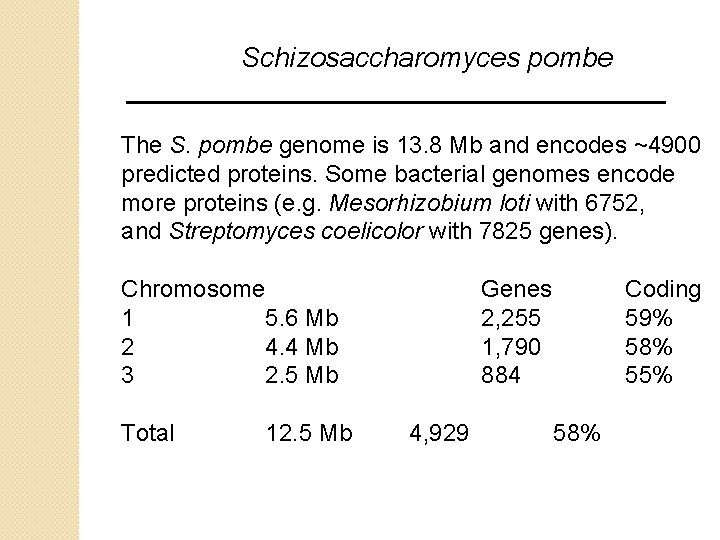
Schizosaccharomyces pombe The S. pombe genome is 13. 8 Mb and encodes ~4900 predicted proteins. Some bacterial genomes encode more proteins (e. g. Mesorhizobium loti with 6752, and Streptomyces coelicolor with 7825 genes). Chromosome 1 5. 6 Mb 2 4. 4 Mb 3 2. 5 Mb Total 12. 5 Mb Genes 2, 255 1, 790 884 4, 929 Coding 59% 58% 55% 58%

Schizosaccharomyces pombe S. pombe diverged from S. cerevisiae about 330 to 420 million years ago. Many genes are as divergent between these two fungi as they are diverged from humans.

Outline Introduction Description and classification of fungi Introduction to budding yeast Saccharomyces cerevisiae Gene duplication and genome duplication Comparative analyses of hemiascomycetes Analysis of fungal genomes Perspective

Perspectives The budding yeast S. cerevisiae is one of the most significant organisms in biology: • Its genome is the first of a eukaryote to be sequenced • Its biology is simple relative to metazoans • Through yeast genetics, powerful functional genomics approaches have been applied to study all yeast genes It is important to note that even for yeast, our knowledge of basic biological questions is highly incomplete. For

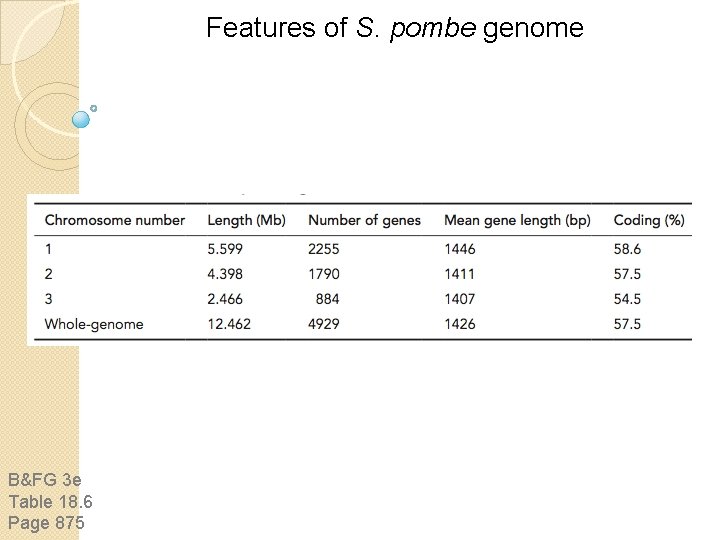
Features of S. pombe genome B&FG 3 e Table 18. 6 Page 875