Chapter 16 The Food and Drug Administration FDA










![FDA History Continued • => 1976 Medical Device Amendments [new=> review, old=>grandfathered, new equivalent=>grandfathered] FDA History Continued • => 1976 Medical Device Amendments [new=> review, old=>grandfathered, new equivalent=>grandfathered]](https://slidetodoc.com/presentation_image_h/1c22a5663f22e28831192f5e15f322b0/image-11.jpg)








![Device Classification: Class III • Life sustaining • 510 k => IDE => [data!!!] Device Classification: Class III • Life sustaining • 510 k => IDE => [data!!!]](https://slidetodoc.com/presentation_image_h/1c22a5663f22e28831192f5e15f322b0/image-20.jpg)


















- Slides: 39

Chapter 16 The Food and Drug Administration

FDA Prehistory • 1862, President Lincoln appointed first director of Department of Agriculture • 1883 Request for petition to pass laws prohibiting adulteration & misbranding of foods and drugs. • For serious drinking, the drugstore was the place to go. Lydia E. Pinkham's Vegetable Compound, a popular nostrum for women, was depending on the formula, 15 to 20 percent alcohol (VU).

Advertising Cards • A common method for medicine-makers to hawk their products was to provide druggists with postcard-sized advertisements with a picture on one side and a description of the glories of the product on the other. • Ayer's Sarsaparilla • Dr. Morse's Indian Root Pills • Hires' Root Beer • Lydia E. Pinkham's Vegetable Compound (First Card) • Lydia E. Pinkham's Vegetable Compound (Second Card) • Pond's Extract • Rumford Yeast Powder • Wells, Richardson, & Co. 's Lactated Food http: //www. mc. vanderbilt. edu/biolib/hc/nostrums/cards. html

The Jungle, Upton Sinclair, 1906 …with the hot weathere descended upon Packingtown a veritable Egyptian plague of flies; there could be no describing this--the houses would be black with them. There was no escaping; you might provide all your doors and windows with screens, but their buzzing outside would be like the swarming of bees, and whenever you opened the door they would rush in as if a storm of wind were driving them. (Chapter 10)

FDA History • 1906: FDA formed, banned interstate commerce in adulterated misbranded food, drink, and drugs • 1938 107 deaths of (primarily) children due to ingestion of Elixir of Sulfanilamide, a toxic combination of diethylene glycol and sulfa. • =>1938 Federal Food, Drug and Cosmetic Act

FDA Act 1938 • requiring that new drugs be shown to be safe before marketing. • extending FDA’s control to cosmetics and therapeutic devices. • authorizing factory inspections and standards of identity for food staples. • eliminating a requirement to prove intent to defraud in drug misbranding cases. • adding court injunctions to the previous penalties of seizures and prosecutions

Drug Testing Guidelines: 1. Chemical Composition 2. Acute toxicity studies in two species 3. Chronic toxicity studies with different doses in different species 4. Observation of animals 5. Pathological examination of animals 6. Studies on the absorption and elimination of the chemical 7. Study of interactions 8. Knowledge of idiosyncrasies and untoward reactions

http: //www. museumofquackery. com Get Rid of the Spectacle Nuisance Regain Your Eye Freedom SIMPLY-SATISFACTORILY-SAFELY The Natural Eyesight System Tells You How No Doctoring-No Drugs-No Danger Nothing to Wear and Nothing to Fear

The Crystaldyne Pain Reliever, 1996 Similar to The Stimulator is essentially an electric gas barbecue grill igniter outfitted with finger grips. When pressed against the skin, the devices sparks and causes a small electric shock. Makers of the device claim it can relieve headaches, back pain, arthritis, stress, menstrual cramps, earaches, sinus, nosebleeds, flu and other ailments. Because of its medical claims, the Stimulator is considered a medical device. The manufacturers, however, have not complied with any FDA regulations that govern the marketing of medical devices. The companies have submitted no information to FDA showing that the device is either safe or effective. . .

FDA History Continued • 1962 sale/use of thalidomide banned in U. S. based upon thousands of birth defects, primarily in Europe & South America – amendment for efficacy & safety • 1969 – hearings re injuries (10, 000) & deaths (731) in a 10 year period [heart valves, pacemakers, IUDs, … (Nader)]
![FDA History Continued 1976 Medical Device Amendments new review oldgrandfathered new equivalentgrandfathered FDA History Continued • => 1976 Medical Device Amendments [new=> review, old=>grandfathered, new equivalent=>grandfathered]](https://slidetodoc.com/presentation_image_h/1c22a5663f22e28831192f5e15f322b0/image-11.jpg)
FDA History Continued • => 1976 Medical Device Amendments [new=> review, old=>grandfathered, new equivalent=>grandfathered] • =>1978 Good Manufacturing Practices • =>1984 Medical Device Reporting • =>1988 Device Recall/Rebuild • =>1990 Safe Medical Devices

FDA History Continued • =>1992 Medical Device Amendments [tracking, surveillance, reporting, refund, . . ] • =>1997 Modernization Act: …public health is a responsibility shared by the entire health care community. The law directs the agency to carry out its mission in consultations and cooperation with all FDA stakeholders, including consumer and patient groups, the regulated industry, health care professionals, and FDA’s regulatory counterparts abroad…

Labeling …FDA (also) regulates the labeling of products under its jurisdiction. This information, which must be rigorously truthful, well documented, and not misleading, plays a major role in protecting consumers and the public health. The FDAregulated food label is helping shoppers eat a healthy diet; the labeling of drugs and medical devices gives prescribers and patients reliable guidance about the safety and effectiveness of health care products.

Recent TV Ad (October 2001) • • (several photos of couples, …) 3 bottles of male virility compounds! Free three month supply, 3 bottles! Simply pay $8. 50 shipping and handling charges for each bottle! • No health claims are made for these pills in accordance with FDA regulations.

A medical device is: "an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is: - recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them, - intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or - intended to affect the structure or any function of the body of man or other animals, and which does not achieve any of it's primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes. " FDA Definition

Is My Device a Medical Device? • http: //www. fda. gov/Medical. Devices/Device. Regulationand Guidance/default. htm Contains overviews, marketing information, search terms, etc.

Special Terms • 510 k Premarket notification • Exemptions – equivalent to pre 1976 devices or other approved devices • IDE – investigational device exemption • PMAA – premarket approval application • GLP – good laboratory practices • GMP – good manufacturing practices • Design Control • IRB Institutional review board

Device Classification: Class I • • Not life sustaining No risk No fixed standards 510 k (some) => registration => listing => GMP (some) => Record keeping • Tongue depressors, stethoscopes*, screwdrivers, …

Device Classification: Class II • Not life sustaining, but must meet control/performance standards • 510 k => IDE => PMAA => Registration => Record keeping • Sphygmanometer, powered wheelchairs, drapes, infusion pumps
![Device Classification Class III Life sustaining 510 k IDE data Device Classification: Class III • Life sustaining • 510 k => IDE => [data!!!]](https://slidetodoc.com/presentation_image_h/1c22a5663f22e28831192f5e15f322b0/image-20.jpg)
Device Classification: Class III • Life sustaining • 510 k => IDE => [data!!!] => PMAA => Registration => Record Keeping • Data & techniques must include failure mode analysis, animal test, toxicology studies, human trials (IRB), PDP (product development protocol. )

FDA approval? • One or more years • Now you will be monitored…

Registration • (All US (foreign optional))+ (manufacturer or preparer or processor or propagator of device) must register yearly with FDA & get a device registration #. (30 days change)

Listing • All manufacturers must list w FDA all devices they market (q 6 mo. ) • All manufacturers must supply FDA with all labeling & advance copy of new labeling.

Registration + Listing • = traceability & recallability • FDA may force recall, fines, reparations, repair, … • FDA may inspect devices, records, labeling, documentation, licenses, laboratories, premises, etc at virtually any time. • FDA requires reporting of adverse events (MAUDE system. )

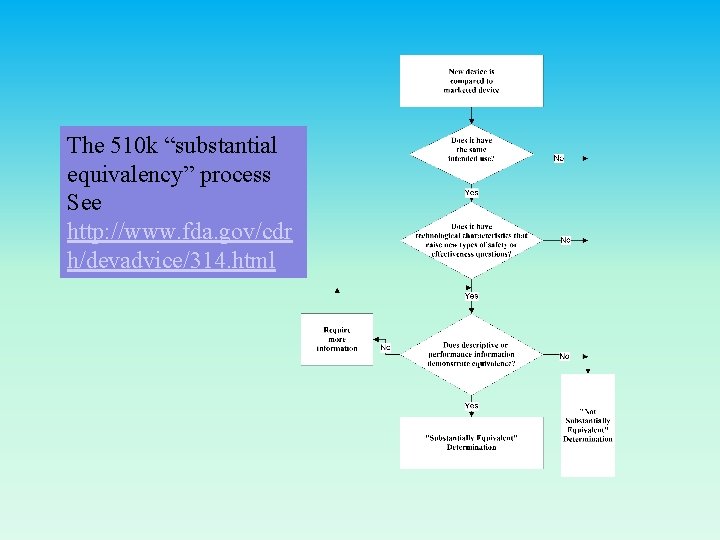
The 510 k “substantial equivalency” process See http: //www. fda. gov/cdr h/devadvice/314. html

Related 510 ks • Special – minor modification of current device • Abbreviated – guidance/control/consensus in place

Declaration of Conformance to a Recognized Standard • ID of standards that were met • ID of any variations (alternate steps) • ID of any standards not applicable

Premarket Approval • Class 3, ensures adequate review • Fairly fixed format/content, includes standards, investigations, samples, all relevant data…

Investigational Device Exemptions • Must have IRB approval(s) [equitable risk & selection, informed consent (documented), adequate safety controls, privacy/confidentiality…] • Investigator is RESPONSIBLE • No fixed format …

Good Laboratory Practices • 1978 laws requiring GLP and laboratory audit and inspection procedures by the FDA… • Must have records for audit, proof of maintenance, evaluation, inspection, etc.

Good Manufacturing Practices • FDA requirements regarding manufacturing of medical devices • Auditable records must be kept re input/output/QA/record keeping/standards/inspections/etc… • FDA may inspect/fine/etc.

Human Factors • Guidance document (1996) – “Do it by design. ” see http: //www. fda. gov/cdrh/humfac/doit. html • General discussion/guidance re the design of medical devices • May become a required part of device documentation…

Design Controls • See http: //www. fda. gov/cdrh/comp/designgd. ht ml • General guidance document regarding the application of design controls/methods that can properly address risk management, design & development planning, interfaces, design cycles, etc.

Software • If part of a device, generally must meet same minimum standards as the device. • If standalone (say drug calculator) same general levels: I = no risk, II = some, esp. if in radiation therapy machines, III = life sustaining • 8 -20% of all deaths are software related. • FDA has published guidelines, can inspect…

FDA Inspections • Access to everything but sales/pricing/financial data • Close/fine/recall/etc. powers… • Cooperate! Keep up to date on regulations! Have a good QA program…

Drugs • FDA has even more say here. • All drugs must be tested on at least 2 properly chosen animal models (thalidomide). (Generally do test tube toxicity test first, then animals. ) • Testing on humans must go through three or more stages under IRB control. Testing must stop if there is any untoward event. • Powers: fine, recall, inspect, etc.

Animals? • Chimpanzees, cats, dogs, mice/rats**, primates, rabbits, pigs, sheep, ferrets, woodchuck, armadillo, guinea pigs, lobsters, chinchillas, electric eels, opossums, angler fish, axolotl, slugs, pigeons, shark, zebra fish, tropical fish, trout, goldfish, C elegan, fruit fly, etc.

Drug testing in Humans • Under IRB approval: (Informed consent) • Phase 1: small cohort, 20 -80 people, look for major problems, safety, tolerance, dose limiting toxicities, mode of entry, dose range • Phase 2: 100 - 300, look for dosing concerns, look for minor problems, efficacy, safety • Phase 3: 1000 -3000 develop and prove statistics on efficacy, safety, tolerance, possible other uses.

FDA Approval? • Screen 5000 compounds => 5 trials => 1 drug…. • Blind/Double blind studies… • High cost of development ($360 M), giving rise to mathematical modeling & simulation… • Penalties! $100 M fine – Abbott Labs Q/A. • ……………. questions?
 Jordan food and drug administration
Jordan food and drug administration Local route of drug administration
Local route of drug administration Oral route advantages
Oral route advantages Local route of drug administration
Local route of drug administration Define pharmacology
Define pharmacology What are the different routes of drug administration
What are the different routes of drug administration Non parenteral route
Non parenteral route Drug administration definition
Drug administration definition Factors affecting choice of route of drug administration
Factors affecting choice of route of drug administration Mydriatics and miotics drugs
Mydriatics and miotics drugs An example of crude drug adulterated with exhausted drug
An example of crude drug adulterated with exhausted drug Unit 2 food food food
Unit 2 food food food Eltonian pyramid
Eltonian pyramid National institute for food and drug surveillance
National institute for food and drug surveillance Muckrakers definition
Muckrakers definition Food drug
Food drug Fda v brown and williamson
Fda v brown and williamson Linux operation and administration chapter 8
Linux operation and administration chapter 8 Chapter 17 dosage calculation and medication administration
Chapter 17 dosage calculation and medication administration Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Chúa yêu trần thế
Chúa yêu trần thế Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau





























































