Overview of the Food and Drug Administration FDA





















- Slides: 29

Overview of the Food and Drug Administration (FDA) & the Office of Orphan Products Development (OOPD) Gayatri R. Rao, MD, JD Acting Director Office of Orphan Products Development FDA Rare Disease Patient Advocacy Day March 1, 2012 1

Outline • • FDA History Modern FDA Product Development 101 Overview of Office of Orphan Products Development (OOPD) 2

Early History of FDA • Origin of FDA was Bureau of Chemistry in the US Department of Agriculture (USDA) • Dr. Harvey Washington Wiley – Chief Chemist, Bureau of Chemistry (USDA), appointed in 1882 • Precursor to Commissioner of FDA • “Poison Squad” – Consisted of ~12 men, mostly from USDA – Ate variety of chemical preservatives & coloring agents (e. g. , borax, copper sulfate, formaldehyde, saccharine) in increasing doses to demonstrate deleterious effects 3

1906 Pure Food and Drug Act (Wiley Act) • Applied to food and drugs • Prohibited false or misleading labeling – Had an enforcement mechanism for misbranding • Created food and drug inspection programs • No premarket safety or effectiveness requirements • USDA charged with implementation 4

5

Elixir of Sulfanilamide (1937) • Sulfa drugs – good anti-infective but only available in bulky tablet/capsule – Increased demand for liquid form • Manufacturer used diethylene glycol (DEG), an analogue to anti-freeze, as a solvent – No animal testing or literature search to verify safety • 107 deaths (many children) in 15 states • Only violation: Misbranding – Elixir by definition contains alcohol – Called an elixir but did not contain alcohol 6

1938 Federal Food, Drug and Cosmetic Act • Pre-market safety requirements – Application submission • Sponsors of new drugs submit safety data to FDA before marketing • Default review system - 60 days • Required listing of active ingredients with adequate directions for use • Prohibited false therapeutic claims • Regulation of therapeutic devices and cosmetics • Enhanced enforcement mechanisms 7

Thalidomide (Kevadon) • 1950’s – Introduced in Europe as a tranquilizer – Later marketed as sleeping pill to ease morning sickness in early pregnancy • 1960’s – Found to cause severe birth defects (e. g. , flipper-like limbs, deafness, blindness, etc. ) • Dr. Frances Kelsey (1960), FDA medical officer – Assigned to review a drug application submitted by an American licensee – Did not approve the drug for marketing in the US • Needed more safety studies for prolonged use and use in pregnancy 8 http: //www. bbc. co. uk/news/magazine-15536544

Kefauver-Harris Amendments of 1962 • Submit safety and effectiveness data to FDA prior to marketing • Good manufacturing practice (GMP) • Adverse event reporting (post-approval) • Regulation over prescription drug advertising • Informed consent for investigational drug trials 9

Medical Device Amendments of 1976 • Dalkon Shield (IUD)marketed in 1971 – Wicking string caused an increased risk of developing Pelvic Inflammatory Disease – FDA pulled the device off the market in 1973 • Ensure the safety and effectiveness of medical devices – Created, among other things, the premarket approval pathway 10

Other Relevant Major Legislation • Orphan Drug Act of 1983 (ODA) • Drug Price Competition and Patent Term Restoration Act • User Fee Related Legislation – Prescription Drug User Fee Act (PDUFA) – Medical Device User Fee and Modernization Act (MDUFMA) • Food and Drug Administration Modernization Act (FDAMA) • Best Pharmaceuticals for Children Act • Pediatric Research Equity Act 11

What is the modern FDA? FDA evolved from a purely scientific agency to a consumer protection agency with a public health mission RISKS BENEFITS 12

What is within FDA’s purview? • Foods, except for most meat and poultry products, which are regulated by USDA • Food additives • Infant formulas FDA regulates • Dietary supplements ~$1 trillion • Human drugs worth of • Vaccines, blood products, and other biologics products • Medical devices each year • Electronic products that emit radiation • Cosmetics • Veterinary feed, drugs, and devices • Tobacco products 13 http: //www. fda. gov/About. FDA/Transparency/Basics/ucm 194879. htm

What is NOT within FDA’s purview? • Some examples: – Reimbursement – Drug pricing – Health insurance – Illegal drugs of abuse – e. g. , heroin, marijuana • FDA often shares regulatory responsibilities for certain products with other government agencies 14 http: //www. fda. gov/About. FDA/Transparency/Basics/ucm 203499. htm

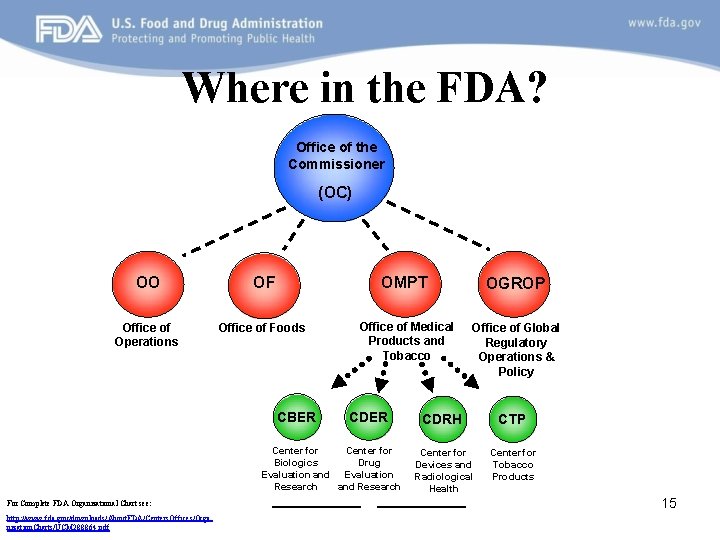
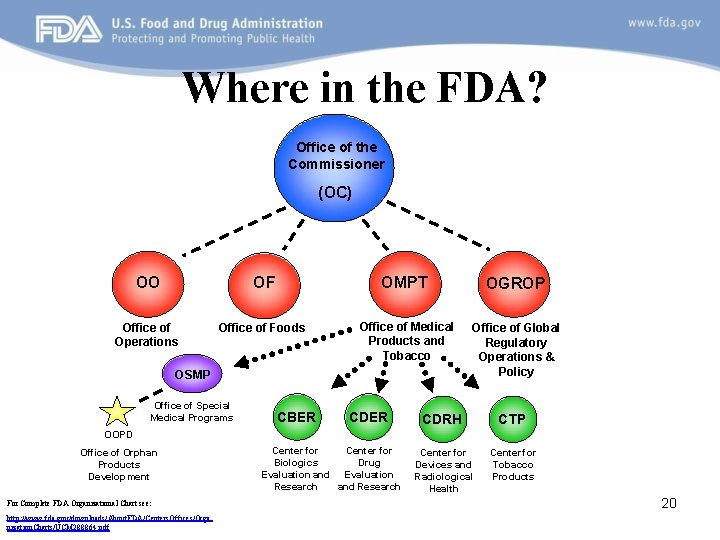
Where in the FDA? Office of the Commissioner (OC) OO OF OMPT OGROP Office of Operations Office of Foods Office of Medical Products and Tobacco Office of Global Regulatory Operations & Policy CBER CDER Center for Biologics Drug Evaluation and Evaluation Research and Research For Complete FDA Organizational Chart see: http: //www. fda. gov/downloads/About. FDA/Centers. Offices/Orga nization. Charts/UCM 288864. pdf CDRH CTP Center for Devices and Radiological Health Center for Tobacco Products 15

Product Development 101 Discovery/Invention Preclinical Development – In vitro – Animal models Clinical Development (Testing in humans) Marketing Application Submission Regulatory Evaluation by FDA Product Launch (if approved) Post-marketing studies 16

Why does it take so long for products to come to market? • Years associated with each stage of development – Inconclusive results in studies • Cost and resources of development • Product manufacturing issues 17

Additional potential road blocks for rare disease product development • Natural history of disease often not well understood • Highly heterogeneous group of disorders – ~7, 000 rare diseases – Difficult to extrapolate from one to another • Small populations – Competing drugs/trials focus on same patient population – Patient enrollment – Difficult to design adequate studies and get meaningful results in studies • Endpoints, outcome measures, tools, instruments, biomarkers usually lacking • Lack of Product 18

The Orphan Drug Act (ODA) • Decade prior to 1983 – only ~1 drug/year independently developed by sponsors for rare diseases – Industry reluctant to invest in small markets • ODA became law on Jan. 4, 1983 – Established the policy that the federal government would assist in development of products for the diagnosis, prevention or treatment of rare diseases or conditions • Patient advocacy brought about the ODA 19

Where in the FDA? Office of the Commissioner (OC) OO OF OMPT OGROP Office of Operations Office of Foods Office of Medical Products and Tobacco Office of Global Regulatory Operations & Policy OSMP Office of Special Medical Programs CBER CDRH CTP Center for Devices and Radiological Health Center for Tobacco Products OOPD Office of Orphan Products Development For Complete FDA Organizational Chart see: http: //www. fda. gov/downloads/About. FDA/Centers. Offices/Orga nization. Charts/UCM 288864. pdf Center for Biologics Drug Evaluation and Evaluation Research and Research 20

Office of Orphan Products Development (OOPD) Programs • Designation Programs (qualify for incentives) – Orphan Drug – Humanitarian Use Device • Grant Programs – Orphan Products Grant Program – Pediatric Device Consortia Grants Program 21

Orphan Drug Designation • What is an orphan drug? – Generally, drug (or biological product) used for the prevention, diagnosis or treatment of a rare disease in the US • What is a rare disease? – Generally, a disease/condition that affects <200 K people in the US • Financial incentives that flow from designation – Tax credits – up to 50% of qualified clinical trial costs – Waiver of user fees – $1. 8 M – 7 -years of marketing exclusivity 22

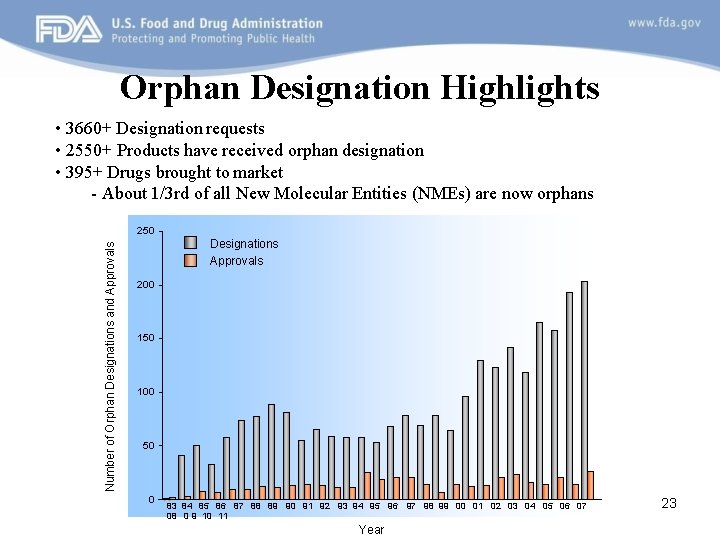
Orphan Designation Highlights • 3660+ Designation requests • 2550+ Products have received orphan designation • 395+ Drugs brought to market - About 1/3 rd of all New Molecular Entities (NMEs) are now orphans Number of Orphan Designations and Approvals 250 Designations Approvals 200 150 100 50 0 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 00 01 02 03 04 05 06 07 08 0 9 10 11 Year 23

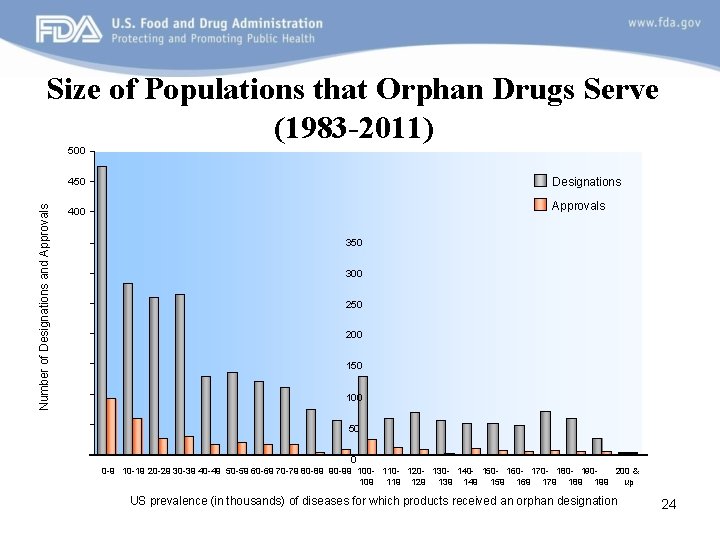
Size of Populations that Orphan Drugs Serve (1983 -2011) Number of Designations and Approvals 500 450 Designations 400 Approvals 350 300 250 200 150 100 50 0 200 & 0 -9 10 -19 20 -29 30 -39 40 -49 50 -59 60 -69 70 -79 80 -89 90 -99 100 - 110 - 120 - 130 - 140 - 150 - 160 - 170 - 180 - 190109 119 129 139 149 159 169 179 189 199 up US prevalence (in thousands) of diseases for which products received an orphan designation 24

Humanitarian Use Device Program • HUD designation granted to a device for use in a disease or condition affecting < 4000 individuals in US/year – If designated, device may be eligible for approval via a Humanitarian Device Exemption (HDE) pathway • >250 HUD requests received; >165 granted HUD designation (~66%) – >50 HDEs approved 25

Grants Program • Orphan Products Grants Program – Clinical development of products for use in treatment of rare diseases or conditions • Drugs, biological products, medical devices, medical foods – Current Annual Budget ~ $14 million – ~2000 applications, over 500 studies funded • >45 products to marketing approval • Pediatric Device Consortia Grants Program – Fund non-profit consortia focused on stimulating pediatric device development – Current Annual Budget – $3 M – 5 pediatric device consortia funded • >90 devices supported 26

OOPD Interactions Within and Outside the Agency • Outside FDA – Patient organizations – Other governmental agencies – Industry • Within FDA – Review Divisions (CBER, CDRH) – OSHI – Drug Shortage 27

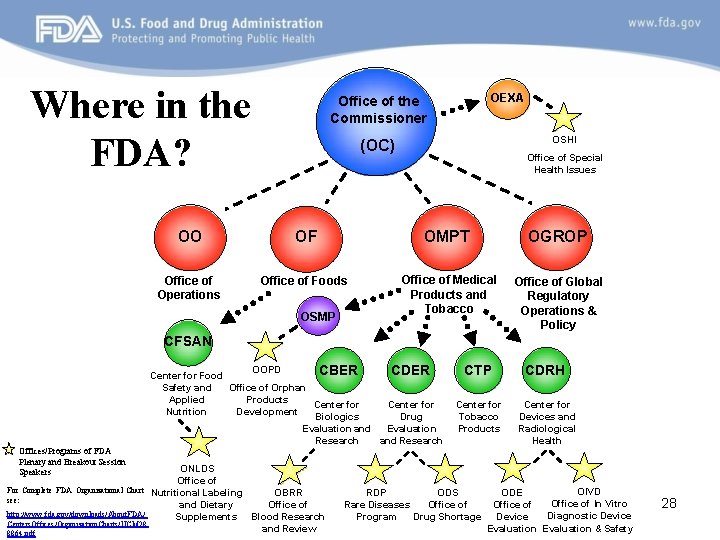
Where in the FDA? OEXA Office of the Commissioner OSHI (OC) Office of Special Health Issues OO OF OMPT OGROP Office of Operations Office of Foods Office of Medical Products and Tobacco Office of Global Regulatory Operations & Policy OSMP CFSAN Offices/Programs of FDA Plenary and Breakout Session Speakers For Complete FDA Organizational Chart see: http: //www. fda. gov/downloads/About. FDA/ Centers. Offices/Organization. Charts/UCM 28 8864. pdf OOPD CBER CDER Center for Food Office of Orphan Safety and Products Applied Center for Development Nutrition Biologics Drug Evaluation and Research ONLDS Office of Nutritional Labeling OBRR and Dietary Office of Supplements Blood Research and Review CTP CDRH Center for Tobacco Products Center for Devices and Radiological Health RDP ODS Rare Diseases Office of Program Drug Shortage OIVD ODE Office of In Vitro Office of Diagnostic Device Evaluation & Safety 28

References 1) http: //www. fda. gov/About. FDA/What. We. Do/History/default. htm 2) http: //www. fsis. usda. gov/about/Agency_History/index. asp 3) http: //www. bbc. co. uk/news/magazine-15536544 4) Pines, W. L. (Ed. ). (2006). A Century of Consumer Protection. Washington, D. C. 29
 Jordan food and drug administration
Jordan food and drug administration Local route of drug administration
Local route of drug administration Intraarterial route
Intraarterial route Parenteral route
Parenteral route First pass effect in pharmacology
First pass effect in pharmacology Factors affecting absorption of drug
Factors affecting absorption of drug Nonparenteral
Nonparenteral Angesid
Angesid Factors affecting choice of route of drug administration
Factors affecting choice of route of drug administration Intraperitoneal route advantages and disadvantages
Intraperitoneal route advantages and disadvantages Exhausted drug
Exhausted drug Unit 2 food food food
Unit 2 food food food Eltonian pyramid
Eltonian pyramid Regulatory agencies
Regulatory agencies Facts about muckrakers
Facts about muckrakers Food drug
Food drug Fda v brown and williamson
Fda v brown and williamson Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worms-breton
Tư thế worms-breton Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ




















































