PGA FDA Food and Drug Administration Fany FloresPastor




























- Slides: 31

PGA - FDA Food and Drug Administration Fany Flores-Pastor Descartes Systems (USA) LLC

Food and Drug Administration • • Introduction Agency Programs Disclaiming Intended Use Codes Entity Types Affirmation of Compliance Important Tips

Introduction • Largest partner agency in charge of overseeing the importing of goods in the US • FDA joined ACE/ITDS and started its Pilot in 2015 • You can disclaim immediately on all commodities and ports. No pilot is required • See CSMS #15 -000852 for details on pilot

Agency Programs • • • BIO (Biologics) COS (Cosmetics) DEV (Medical Devices) DRU (Drugs) FDA (FDA Non-Regulated excluding Medical Devices). Only used for disclaiming. FO 1 (Food with Prior Notice) FO 2 (Food with Prior Notice Requirement Previously Met) FO 3 (Food – No Prior Notice Required) RAD (Radiation) TOB (Tobacco) • VME (Animal Drugs and Devices)

Disclaiming • You can Disclaim Immediately (all commodities) • To Disclaim Include: – Description – Processing Code: - Agency Code • FDA – most cases • NED or RED – for Medical Devices – Disclaim Code: • A – Product Not Regulated by Agency • B – Not Required per Agency Guidance

Intended Use • Conditional: – BIO, DEV, DRU, RAD, TOB • Optional: – COS, FO 1, FO 2, FO 3, VME • See Appendix B for IUC per Agency Program

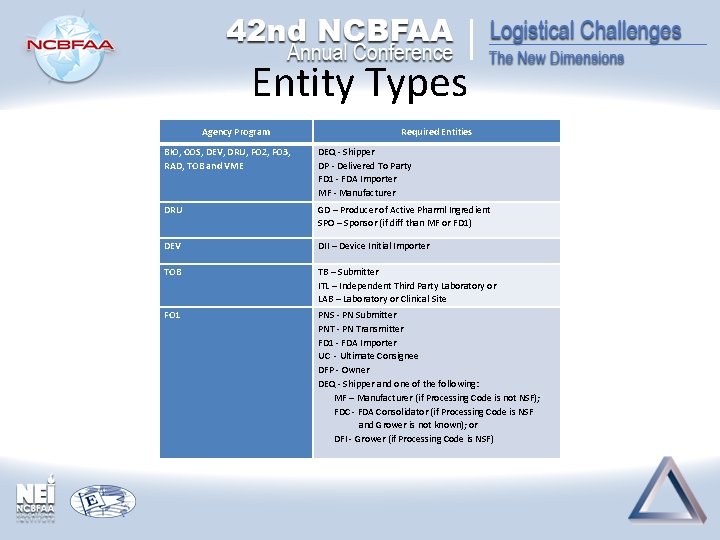
Entity Types Agency Program Required Entities BIO, COS, DEV, DRU, FO 2, FO 3, RAD, TOB and VME DEQ - Shipper DP - Delivered To Party FD 1 - FDA Importer MF - Manufacturer DRU GD – Producer of Active Pharml Ingredient SPO – Sponsor (if diff than MF or FD 1) DEV DII – Device Initial Importer TOB TB – Submitter ITL – Independent Third Party Laboratory or LAB – Laboratory or Clinical Site FO 1 PNS - PN Submitter PNT - PN Transmitter FD 1 - FDA Importer UC - Ultimate Consignee DFP - Owner DEQ - Shipper and one of the following: MF – Manufacturer (if Processing Code is not NSF); FDC - FDA Consolidator (if Processing Code is NSF and Grower is not known); or DFI - Grower (if Processing Code is NSF)

Affirmation of Compliance • Required for: – DEV • Conditional for: – BIO, DRU, FO 1, FO 2, RAD, TOB and VME • Optional for: – COS • See Appendix D for Ao. C per Agency

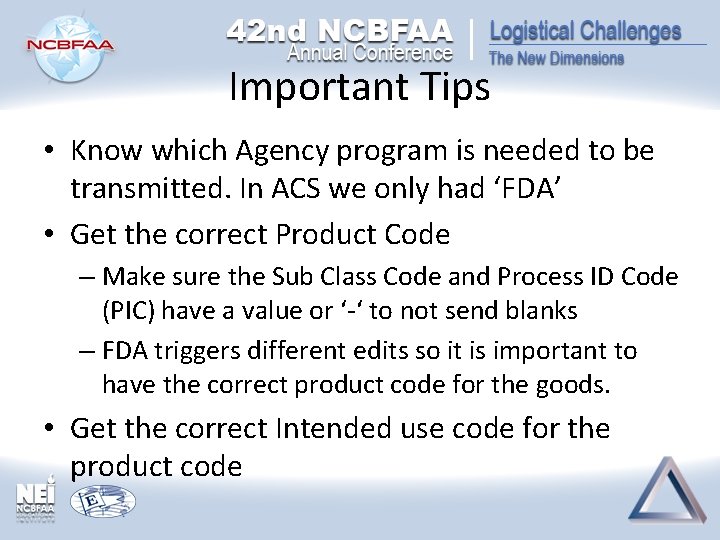
Important Tips • Know which Agency program is needed to be transmitted. In ACS we only had ‘FDA’ • Get the correct Product Code – Make sure the Sub Class Code and Process ID Code (PIC) have a value or ‘-‘ to not send blanks – FDA triggers different edits so it is important to have the correct product code for the goods. • Get the correct Intended use code for the product code

Important Tips – Cont’d • Get the correct Affirmation of Compliance info only send when needed. If you send others, not required, it may trigger errors from FDA • For Entities: – Make sure you submit the required ones for that Agency. For example having the PNT and PNS when doing FDA FOOD with Prior notice. Most require Entry Point of Contact. – Make sure they have an phone, name, and email information.

Important Tips – Cont’d • FDA recently announced the relax on edits for the following required fields, as of April 4, 2016: – Intended Use Code – Brand Name – Device Listing Number (LST) – Active Ingredient Producer (entity code “GD”) • You can enter “UNK” (unknown) instead. • Entity Code “GD” is temporarily not required

Christopher Springer Questa. Web Inc.

• Dates – July 14 TH 1994 - CSMS #94 -000704 – March 31 st 2016 – ACE Mandatory – May 28 th 2016 – No new mandatory PGA’s – Summer 2016 – PGA’s … All Aboard

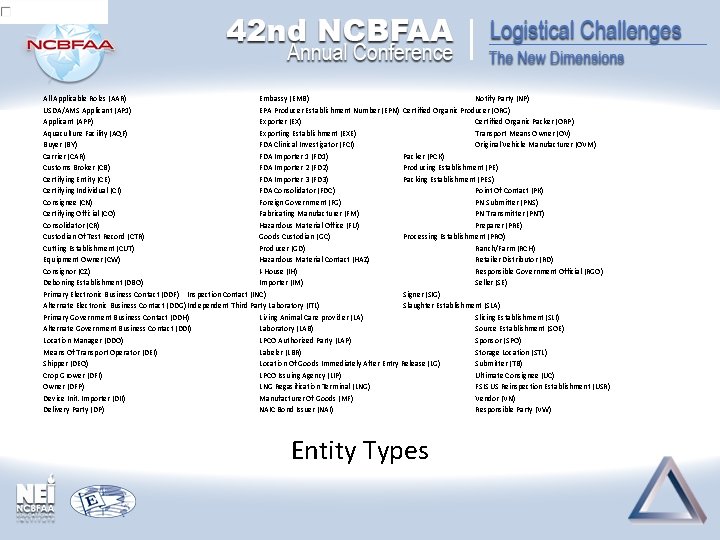
All Applicable Roles (AAR) Embassy (EMB) Notify Party (NP) USDA/AMS Applicant (AP 1) EPA Producer Establishment Number (EPN) Certified Organic Producer (ORG) Applicant (APP) Exporter (EX) Certified Organic Packer (ORP) Aquaculture Facility (AQF) Exporting Establishment (EXE) Transport Means Owner (OV) Buyer (BY) FDA Clinical Investigator (FCI) Original Vehicle Manufacturer (OVM) Carrier (CAR) FDA Importer 1 (FD 1) Packer (PCK) Customs Broker (CB) FDA Importer 2 (FD 2) Producing Establishment (PE) Certifying Entity (CE) FDA Importer 3 (FD 3) Packing Establishment (PES) Certifying Individual (CI) FDA Consolidator (FDC) Point Of Contact (PK) Consignee (CN) Foreign Government (FG) PN Submitter (PNS) Certifying Official (CO) Fabricating Manufacturer (FM) PN Transmitter (PNT) Consolidator (CR) Hazardous Material Office (FU) Preparer (PRE) Custodian Of Test Record (CTR) Goods Custodian (GC) Processing Establishment (PRO) Cutting Establishment (CUT) Producer (GD) Ranch/Farm (RCH) Equipment Owner (CW) Hazardous Material Contact (HAZ) Retailer Distributor (RD) Consignor (CZ) I-House (IH) Responsible Government Official (RGO) Deboning Establishment (DBO) Importer (IM) Seller (SE) Primary Electronic Business Contact (DDF) Inspection Contact (INC) Signer (SIG) Alternate Electronic Business Contact (DDG) Independent Third Party Laboratory (ITL) Slaughter Establishment (SLA) Primary Government Business Contact (DDH) Living Animal Care provider (LA) Slicing Establishment (SLI) Alternate Government Business Contact (DDI) Laboratory (LAB) Source Establishment (SOE) Location Manager (DDO) LPCO Authorized Party (LAP) Sponsor (SPO) Means Of Transport Operator (DEI) Labeler (LBR) Storage Location (STL) Shipper (DEQ) Location Of Goods Immediately After Entry Release (LG) Submitter (TB) Crop Grower (DFI) LPCO Issuing Agency (LIP) Ultimate Consignee (UC) Owner (DFP) LNG Regasification Terminal (LNG) FSIS US Reinspection Establishment (USR) Device Init. Importer (DII) Manufacturer Of Goods (MF) Vendor (VN) Delivery Party (DP) NAIC Bond Issuer (NAI) Responsible Party (VW) Entity Types

• Lacey act (The original PGA) – Was a relatively easy transition – Deviation from ACS CATAIR • Units of measure - M, M 2, M 3 and KG • Entity Information • Declaration Certification

• NHTSA – HS 7 form based – Was challenging with much more data required • • Program codes Intended use codes Category types and codes Entity information – Part file based solutions

EPA form transition to ACE submission PGA Message Set • • • EPA 3520 -1 - Importation of Motor Vehicles and Engines (on road) EPA 3520 -21 - Importation of Motor Vehicles and Engines (non road) EPA - None Pre-approved Vehicle/ Engine Exemption Number EPA 3540 -1 - Notice of Arrival of Pesticides and Devices (NOA) EPA No form - Toxic Substances Control Act (TSCA) Import Certifications DIS Solution • EPA No-form - Pesticide Label • Non-Objection Notice - Used Ozone Depleting Substances Non-Objection Notice


ACE and PGA’s Presented by Craig Seelig Wise. Tech Global Inc NCBFAA April 19, 2016

APHIS Core • • Not same as Lacey Act HTS are flagged AQ 1, AQ 2 Covers everything from live animals, seeds, fruits and vegetables, animal products and byproducts, to flowers and greenery Have to translate the date from the various certificates into the PGA message set

APHIS Core • Very Complex Message Set • PGA Processing Code optional – but might be helpful • Agriculture; Plant Inspection; Veterinary Services; etc • Certificates/Documents • For certificates APHIS has issued, they match it up and can see the document without DIS. (But there is no confirmation of a match. ) • “Health Certificate” can be keyed

ATF • Follow Form 6 • Dashes are now optional • Arms Export Control Act Number • • AECA numbers begin with “A” The format should be: “A-99 -9999” • Permit Number • Format “ 2015 -99999” not 15 -99999 • Federal Firearms License • • Format should be “ 9 -99 -99 -9 A-99999” Hint: Do not call a Magazine “FP” (Firearm parts) if permit lists it as “MAG”

PGA Data Correct (CA) • Tired of cancelling so many entries? Help is coming…. !! • • • Ability to update PGA data Resend ALL PGA data for all PGA’s Resend all PGA data only for changed PGA FDA – still can only do if FDA rejects the data FUTURE – ability to update individual lines

Craig Seelig Product Manager Customs and Complaince Wise. Tech Global Inc Craig. Seelig@wisetechglobal. com

Post Summary Processes Celeste Catano Kewill

ACE Post Summary Processing • • Liquidation Drawback Protest Reconciliation

Liquidation • Liquidations will process weekly on Fridays • Electronic Bulletin notice • System allows for liquidation of unpaid summaries • CBP will not show summaries as liquidated when there is a future anticipated liquidation date

Drawback • Electronic Submission of Entire Drawback Package via ABI or DIS upload • Filing at the 10 digit HTS line level • System Validations for accuracy • Tighter integration with other post release processes • Improved system controls for preventing over refunding.

Protests • All Electronic Protests - filed on the ACE Portal – No more ABI programs – less than 10% usage – Allows for broad range of users • New Protest Filer Account – CEE or Ports can move work as needed – Integration with DIS for supporting documents – Electronic event status (Accepted, Denied, etc. ) – Paper can still be used

Reconciliation • All data through ABI – No more separate reports/data files • 3 types of Reconciliation – No Change (No line item data required) – Change – FTA • No more blanket flagging • “Original” duty/fees/taxes not submitted

Reconciliation • Additional information – FTA Declaration Statement – Classification Pending Action • Protest, Ruling, Court Action – Prior Disclosure Indicator – HTS Change due to Value Indicator – Aggregate Refund Waiver Indicator – Filer Contact Information
 Jordan food and drug administration
Jordan food and drug administration Barnes and noble pga
Barnes and noble pga Drug roa
Drug roa Dermoject
Dermoject Local route of drug administration
Local route of drug administration First pass effect in pharmacology
First pass effect in pharmacology What are the different routes of drug administration
What are the different routes of drug administration Non parenteral
Non parenteral Drug administration definition
Drug administration definition Factors affecting choice of route of drug administration
Factors affecting choice of route of drug administration Intraperitoneal route advantages and disadvantages
Intraperitoneal route advantages and disadvantages Types of adulteration of crude drugs
Types of adulteration of crude drugs Land grid array vs pin grid array
Land grid array vs pin grid array Pga sections map
Pga sections map Pga customs clearance
Pga customs clearance Pga pin grid array
Pga pin grid array Usbdaq
Usbdaq Cobazyn
Cobazyn Unit 2 food food food
Unit 2 food food food Food chain food chain food chain
Food chain food chain food chain National institute for food and drug surveillance
National institute for food and drug surveillance Whats the pure food and drug act
Whats the pure food and drug act Food drug
Food drug Fda v brown and williamson
Fda v brown and williamson Early feasibility study
Early feasibility study Nicole gillette fda
Nicole gillette fda Leonard sacks fda
Leonard sacks fda Real world evidence
Real world evidence Equipment qualification fda
Equipment qualification fda Tocolitico significato
Tocolitico significato Klasyfikacja fda
Klasyfikacja fda Fda klasifikacija lekova u trudnoci
Fda klasifikacija lekova u trudnoci