FDA Medical Device Update US Food and Drug

















- Slides: 26

FDA Medical Device Update US Food and Drug Administration New England District

Position at NWE-DO l l l Supervisory Investigator (May 2002) Monitor for Medical Device Program MDUFMA Coordinator

What is a Program Monitor?

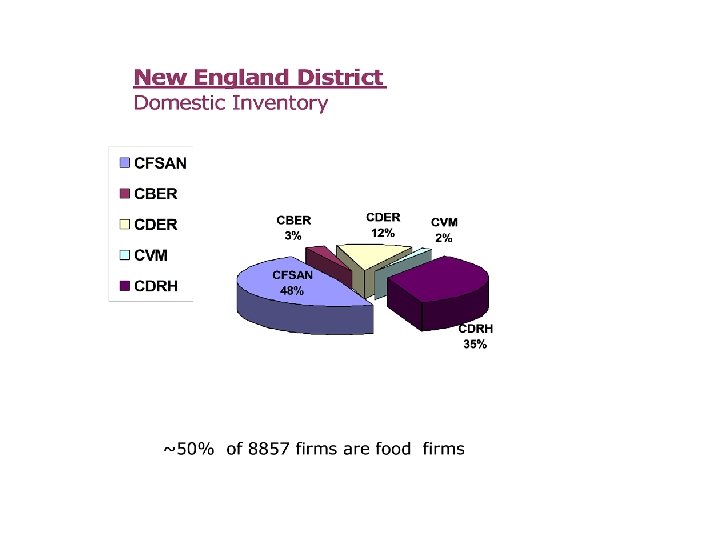
Work for the District is in ~87 different program areas – examples: l Foods – – – l l l Imported cheese Domestic Fisheries Infant formula Juice processing Pesticides …Many more Interstate Travel Dietary Supplements Blood banks l l l Clinical Investigators Drug process inspections New Animal Drug inspections BSE (mad cow) Medical devices (Domestic and foreign) Mammography facilities

Program Monitoring l l All 87 programs are split among the 7 supervisors. Each has 12 -14 programs for which s/he is the monitor

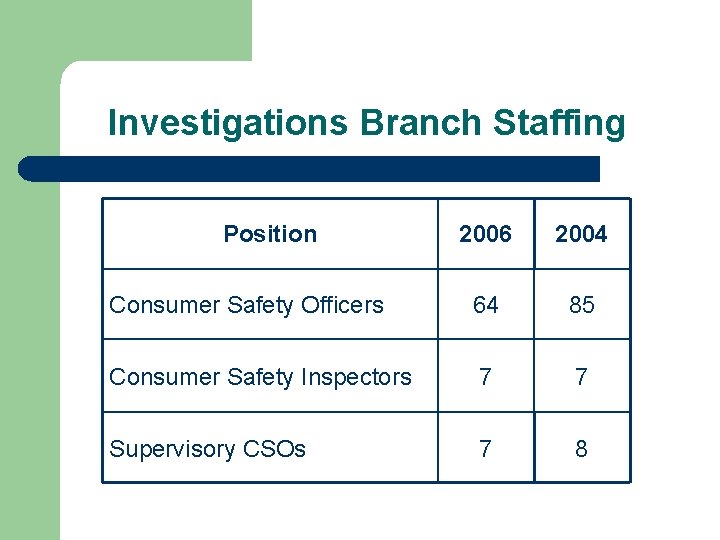
Investigations Branch Staffing Position 2006 2004 Consumer Safety Officers 64 85 Consumer Safety Inspectors 7 7 Supervisory CSOs 7 8

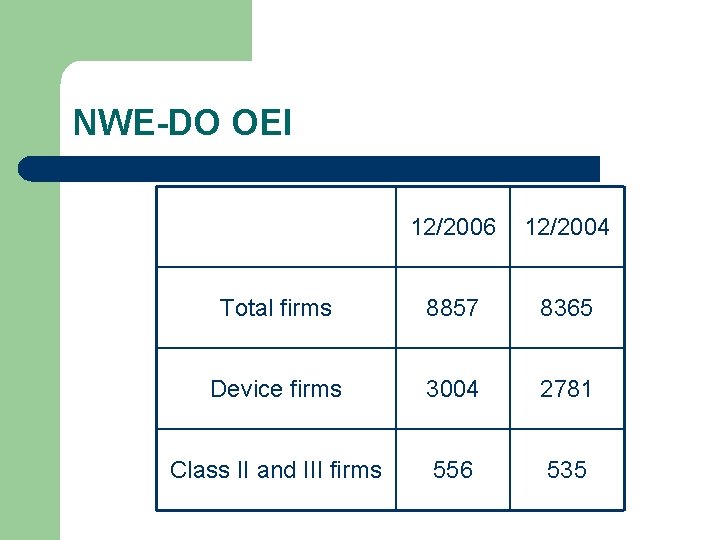
NWE-DO OEI 12/2006 12/2004 Total firms 8857 8365 Device firms 3004 2781 Class II and III firms 556 535


Program monitor role l l l Identify the firms to be inspected or investigated Verify that assignments are created Assure the work is done and properly reported Try to keep OEI up-to-date Summarize accomplishments for management Be the Point of Contact for that program

Device Program – firm selection l l CDRH guidance Workplan – – l Budget Staffing Inspectional History

CDRH Device Inspection Guidance l l l Inspect 20% of Registered Domestic Class II and Class III Medical Device Manufacturers, i. e. , 1, 030. (NWE-DO: 98) Inspect 7% of Registered Foreign Class II and Class III Medical Device Manufacturers, i. e. 168 inspections. Conduct CDRH BIMO Inspections, i. e. , 278 inspections.

CDRH Device Inspection Guidance l l l For Cause MDUFMA – PMA/GMP inspections and other premarket inspections including BIMO Follow-Up to Violative Inspections High/Significant Risk Class III and II Manufacturers Special Emphasis – – Focus on Firms with Repeated Violative Inspections Focus on Risk-Based Center Initiated Inspection Assignments

Risk-based Work Plan Initiative l l CDRH “Call for Proposals”. The Call process incorporates involvement from both Center and Field sources focusing on risks, hazards, justified concerns, and output from various databases to achieve those devices and eventual individual manufacturers, which indicate need for Agency inspectional resources. This is a directed inspection request with specific inspectional guidance in addition to GMP inspection.

FY’ 07 Device Workplan FY 2007 FY 2006 Level I 65 84 Level II 43 53 Compliance f/u 2 8 For Cause 7 9 High Risk Domestic 7 --- Foreign 15 17 AP 2 9 141 180 TOTAL

NWE-DO Staff l l Staff with devices as primary program = 12 Staff with devices as second program = 9

Training activities l l l l 10 CSOs to Basic Medical Device 2 CSOs to process validation 1 CSO to industrial sterilization 1 level II certification audit 2 level II certified 1 auditor certified 1 AP auditor certified

Pre-announcement of Inspection l l Most inspections are pre-announced NOT pre-announced: – – – f/u to Compliance action (W/L, etc. ) f/u to Complaint or informant f/u to observe promised corrections (VAI)

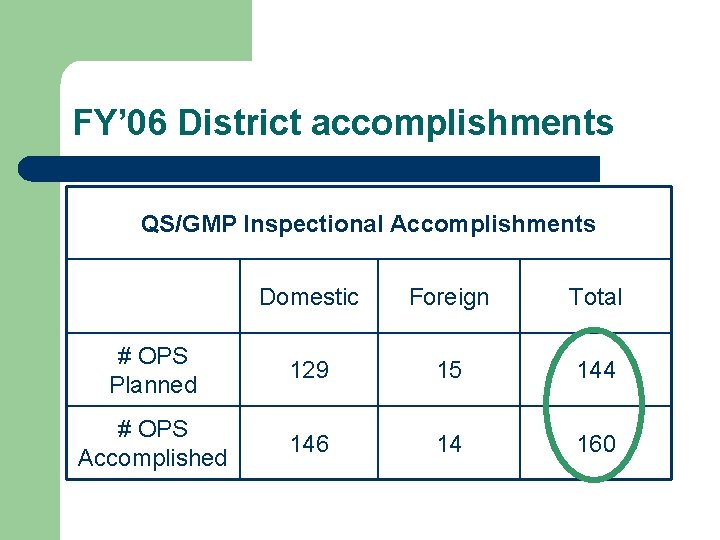
FY’ 06 District accomplishments QS/GMP Inspectional Accomplishments Domestic Foreign Total # OPS Planned 129 15 144 # OPS Accomplished 146 14 160

FY’ 06 Compliance actions l 4 NWE-DO Medical Device firms had EI classified as OAI with Warning letter recommended – 3 Warning letters issued

Summary of FY’ 06 Recalls l l Total recalls in NWE-DO = 212 Total CDRH recalls in NWE-DO = 145 – – – Class 1: 15 Class 2: 116 Class 3: 14

Summary of Consumer Complaints (separate from Med. Watch) l FY’ 05 - 13 Consumer Complaints regarding Medical Devices – l 5 resulted in for-cause inspections FY’ 06 - 13 Consumer Complaints regarding Medical Devices – 3 resulted in for-cause inspections

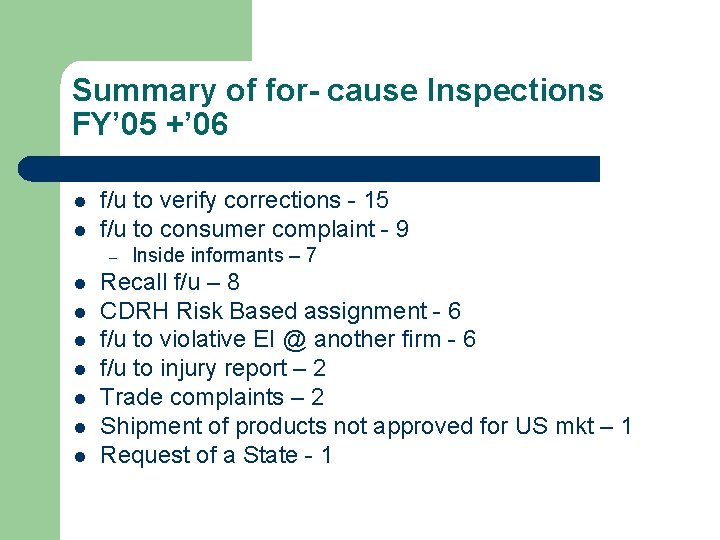
Summary of for- cause Inspections FY’ 05 +’ 06 l l f/u to verify corrections - 15 f/u to consumer complaint - 9 – l l l l Inside informants – 7 Recall f/u – 8 CDRH Risk Based assignment - 6 f/u to violative EI @ another firm - 6 f/u to injury report – 2 Trade complaints – 2 Shipment of products not approved for US mkt – 1 Request of a State - 1

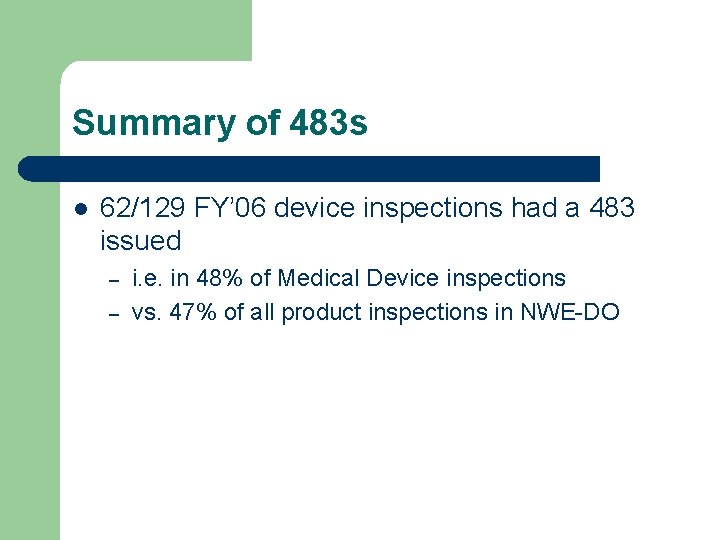
Summary of 483 s l 62/129 FY’ 06 device inspections had a 483 issued – – i. e. in 48% of Medical Device inspections vs. 47% of all product inspections in NWE-DO

Other FY’ 06 NWE-DO Medical Device accomplishments l l l l 3 7 3 6 5 7 50 52 Pre-Market Approval inspections Post-Market Audit inspections Non-clinical studies IRBs (2 U/L) Sponsor/Monitor/CRO (1 W/L) Clinical Investigators Mammography Import Field Exams

Other FY’ 06 NWE-DO Medical Device accomplishments l 2 AP inspections including one level 3 audit – l Candidate failed the audit, but then corrected and passed 2 NWE-DO firms approved for use of third party inspectors under the AP program

Contact Info l l William S. Boivin 781 -596 -7783 (phone) 781 -596 -7896 (fax) william. boivin@fda. gov
 Which is an alternative of log based recovery
Which is an alternative of log based recovery Ram input or output device
Ram input or output device Basic food and beverage knowledge
Basic food and beverage knowledge Exhausted drug meaning
Exhausted drug meaning Halo closed bag adaptor
Halo closed bag adaptor Unit 2 food food food
Unit 2 food food food Food chain sequence
Food chain sequence Regulatory agencies
Regulatory agencies Whats a muckraker
Whats a muckraker Jordan food and drug administration
Jordan food and drug administration Food drug
Food drug Difference between medical report and medical certificate
Difference between medical report and medical certificate Fda vs brown and williamson
Fda vs brown and williamson A tagout device is preferable to using a lockout device.
A tagout device is preferable to using a lockout device. Kelompok output
Kelompok output Gmdn codes
Gmdn codes Medical device academy
Medical device academy Medical device security conference
Medical device security conference Feasibility study medical device
Feasibility study medical device Medical devices software fmea
Medical devices software fmea Cdsco medical device classification
Cdsco medical device classification Vaporizer medical device
Vaporizer medical device Umdns code
Umdns code Medical device safety
Medical device safety Emergo group inc
Emergo group inc California medical license application
California medical license application Gbmc infoweb
Gbmc infoweb