CHAPTER 11 Alkenes Infrared Spectroscopy and Mass Spectroscopy






- Slides: 40

CHAPTER 11 Alkenes; Infrared Spectroscopy and Mass Spectroscopy

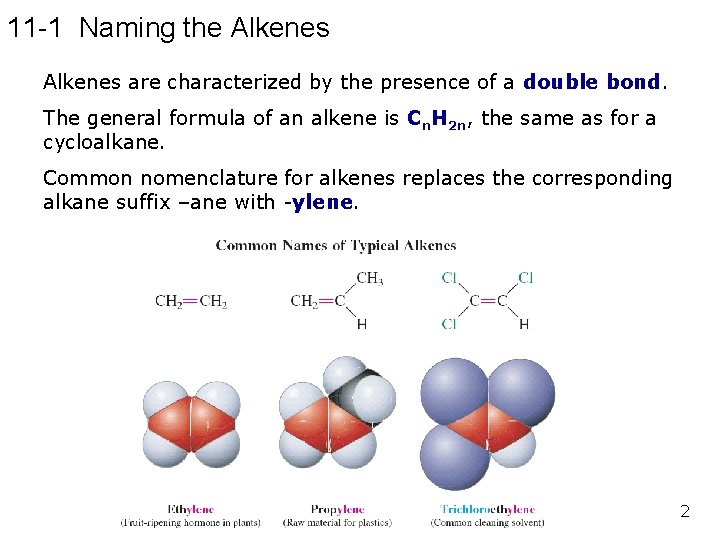
11 -1 Naming the Alkenes are characterized by the presence of a double bond. The general formula of an alkene is Cn. H 2 n, the same as for a cycloalkane. Common nomenclature for alkenes replaces the corresponding alkane suffix –ane with -ylene. 2

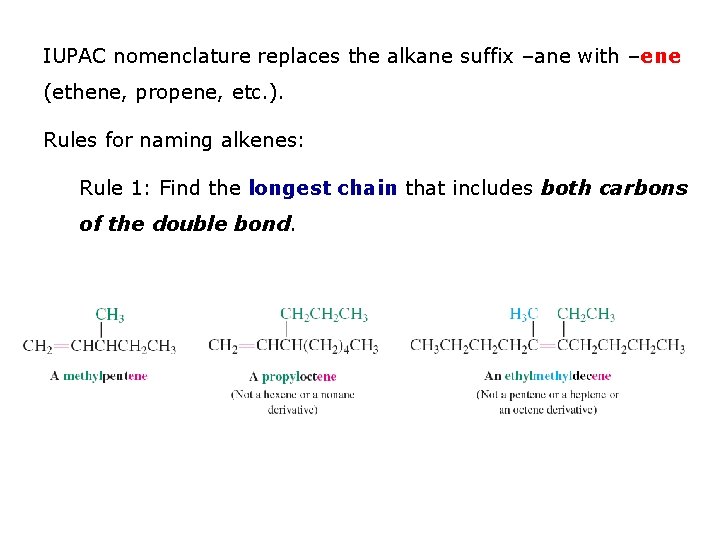
IUPAC nomenclature replaces the alkane suffix –ane with –ene (ethene, propene, etc. ). Rules for naming alkenes: Rule 1: Find the longest chain that includes both carbons of the double bond.

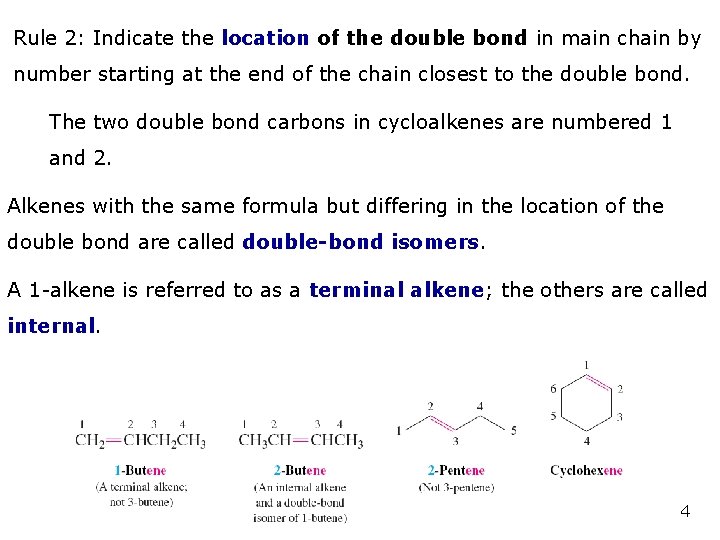
Rule 2: Indicate the location of the double bond in main chain by number starting at the end of the chain closest to the double bond. The two double bond carbons in cycloalkenes are numbered 1 and 2. Alkenes with the same formula but differing in the location of the double bond are called double-bond isomers. A 1 -alkene is referred to as a terminal alkene; the others are called internal. 4

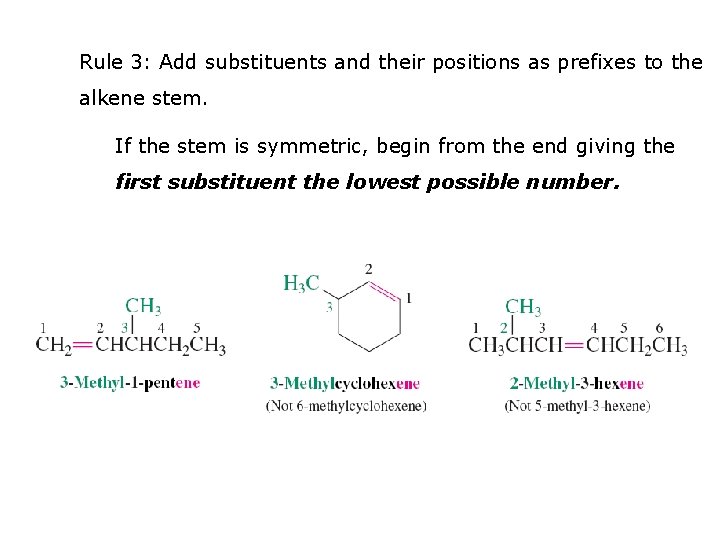
Rule 3: Add substituents and their positions as prefixes to the alkene stem. If the stem is symmetric, begin from the end giving the first substituent the lowest possible number.

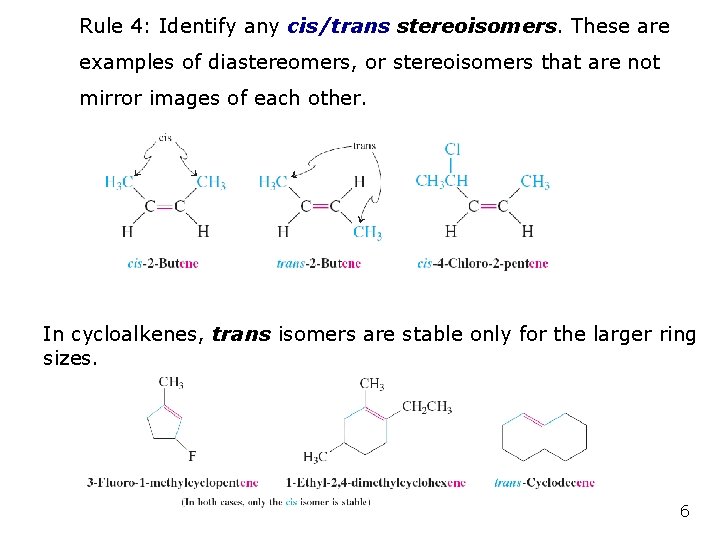
Rule 4: Identify any cis/trans stereoisomers. These are examples of diastereomers, or stereoisomers that are not mirror images of each other. In cycloalkenes, trans isomers are stable only for the larger ring sizes. 6

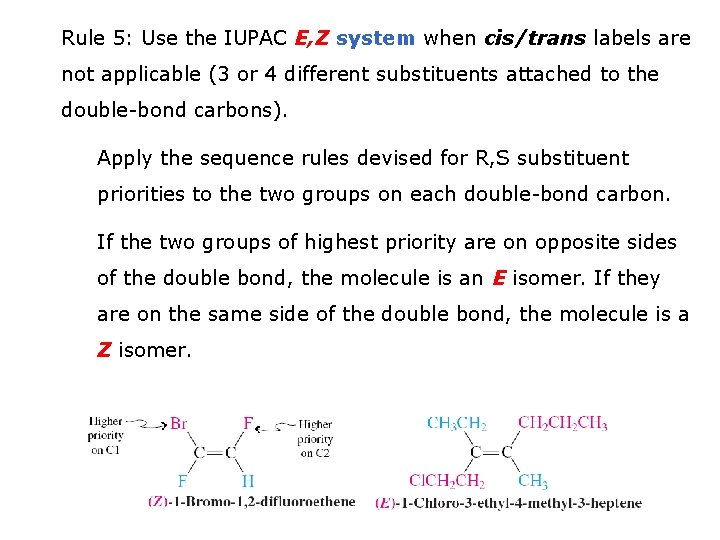
Rule 5: Use the IUPAC E, Z system when cis/trans labels are not applicable (3 or 4 different substituents attached to the double-bond carbons). Apply the sequence rules devised for R, S substituent priorities to the two groups on each double-bond carbon. If the two groups of highest priority are on opposite sides of the double bond, the molecule is an E isomer. If they are on the same side of the double bond, the molecule is a Z isomer.

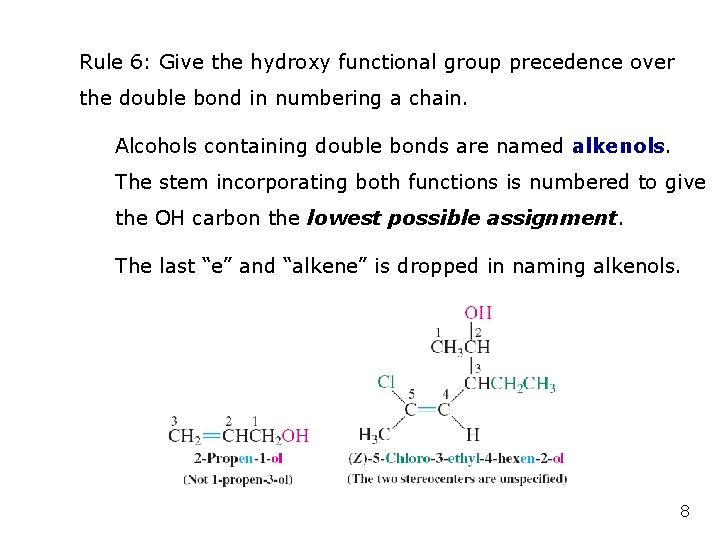
Rule 6: Give the hydroxy functional group precedence over the double bond in numbering a chain. Alcohols containing double bonds are named alkenols. The stem incorporating both functions is numbered to give the OH carbon the lowest possible assignment. The last “e” and “alkene” is dropped in naming alkenols. 8

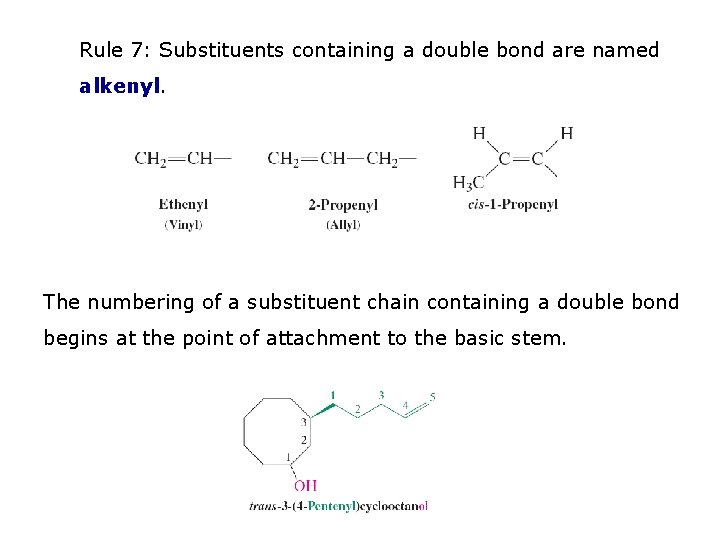
Rule 7: Substituents containing a double bond are named alkenyl. The numbering of a substituent chain containing a double bond begins at the point of attachment to the basic stem.

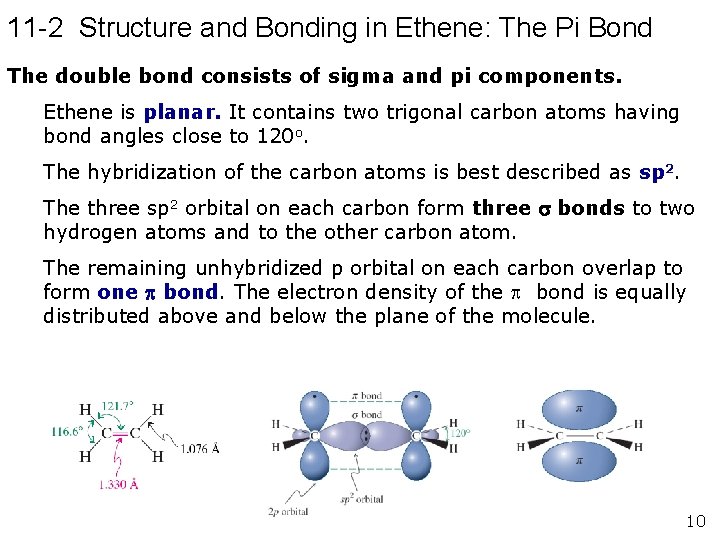
11 -2 Structure and Bonding in Ethene: The Pi Bond The double bond consists of sigma and pi components. Ethene is planar. It contains two trigonal carbon atoms having bond angles close to 120 o. The hybridization of the carbon atoms is best described as sp 2. The three sp 2 orbital on each carbon form three bonds to two hydrogen atoms and to the other carbon atom. The remaining unhybridized p orbital on each carbon overlap to form one bond. The electron density of the bond is equally distributed above and below the plane of the molecule. 10

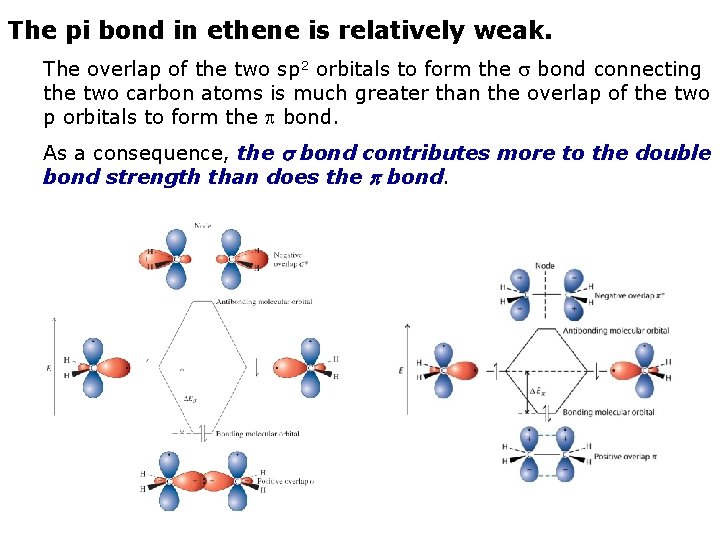
The pi bond in ethene is relatively weak. The overlap of the two sp 2 orbitals to form the bond connecting the two carbon atoms is much greater than the overlap of the two p orbitals to form the bond. As a consequence, the bond contributes more to the double bond strength than does the bond.

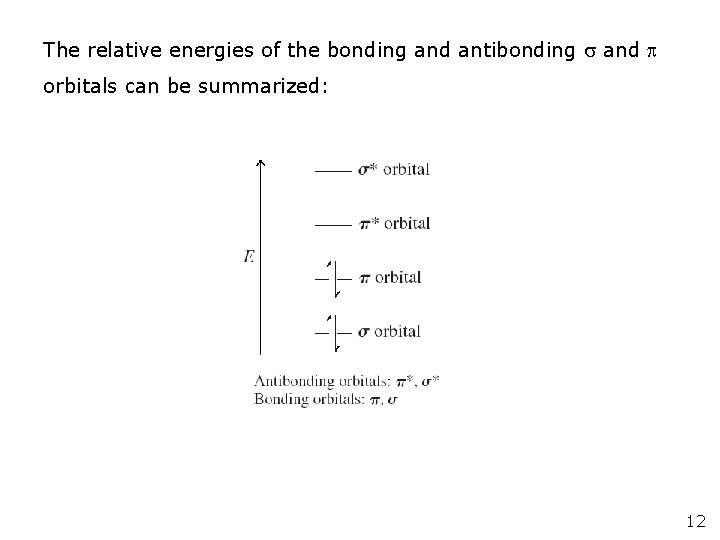
The relative energies of the bonding and antibonding and orbitals can be summarized: 12

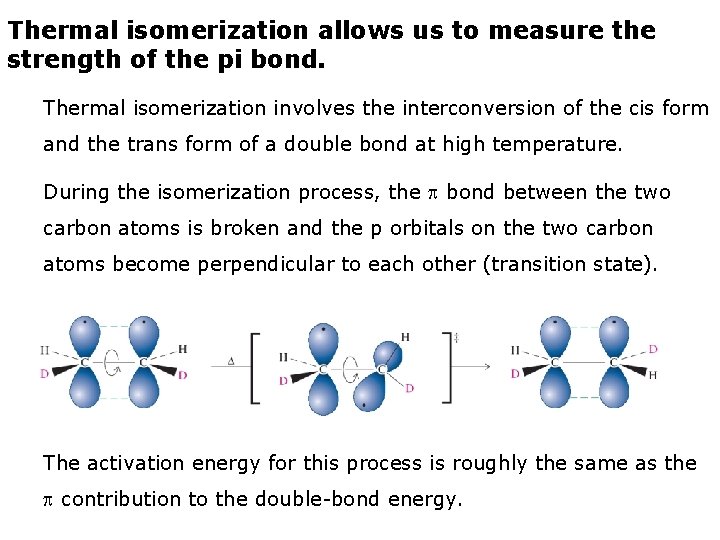
Thermal isomerization allows us to measure the strength of the pi bond. Thermal isomerization involves the interconversion of the cis form and the trans form of a double bond at high temperature. During the isomerization process, the bond between the two carbon atoms is broken and the p orbitals on the two carbon atoms become perpendicular to each other (transition state). The activation energy for this process is roughly the same as the contribution to the double-bond energy.

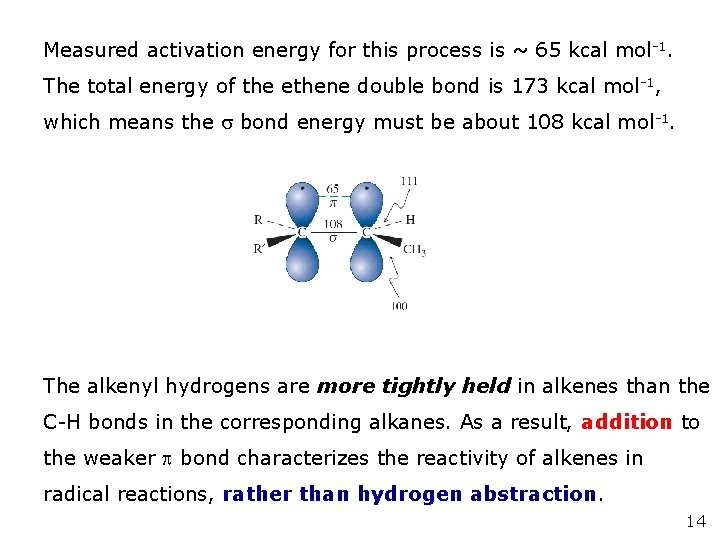
Measured activation energy for this process is ~ 65 kcal mol -1. The total energy of the ethene double bond is 173 kcal mol -1, which means the bond energy must be about 108 kcal mol-1. The alkenyl hydrogens are more tightly held in alkenes than the C-H bonds in the corresponding alkanes. As a result, addition to the weaker bond characterizes the reactivity of alkenes in radical reactions, rather than hydrogen abstraction. 14

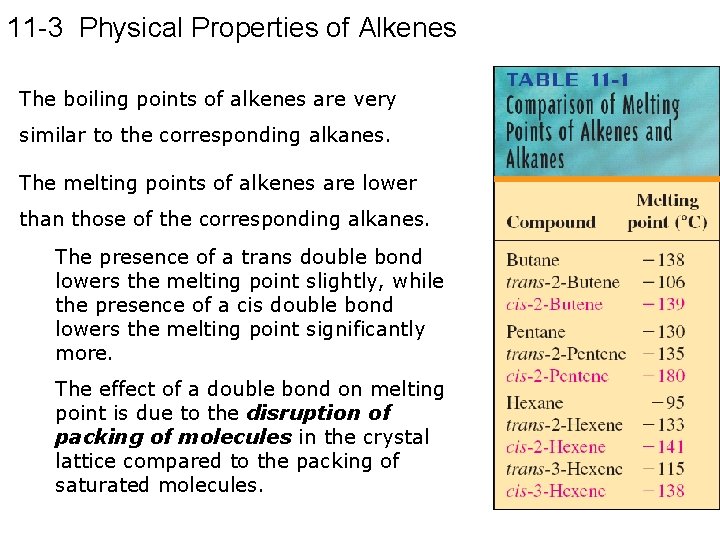
11 -3 Physical Properties of Alkenes The boiling points of alkenes are very similar to the corresponding alkanes. The melting points of alkenes are lower than those of the corresponding alkanes. The presence of a trans double bond lowers the melting point slightly, while the presence of a cis double bond lowers the melting point significantly more. The effect of a double bond on melting point is due to the disruption of packing of molecules in the crystal lattice compared to the packing of saturated molecules.

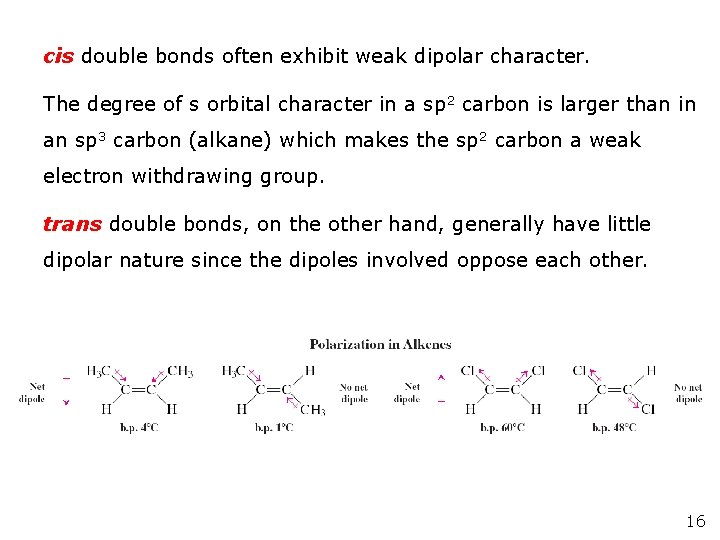
cis double bonds often exhibit weak dipolar character. The degree of s orbital character in a sp 2 carbon is larger than in an sp 3 carbon (alkane) which makes the sp 2 carbon a weak electron withdrawing group. trans double bonds, on the other hand, generally have little dipolar nature since the dipoles involved oppose each other. 16

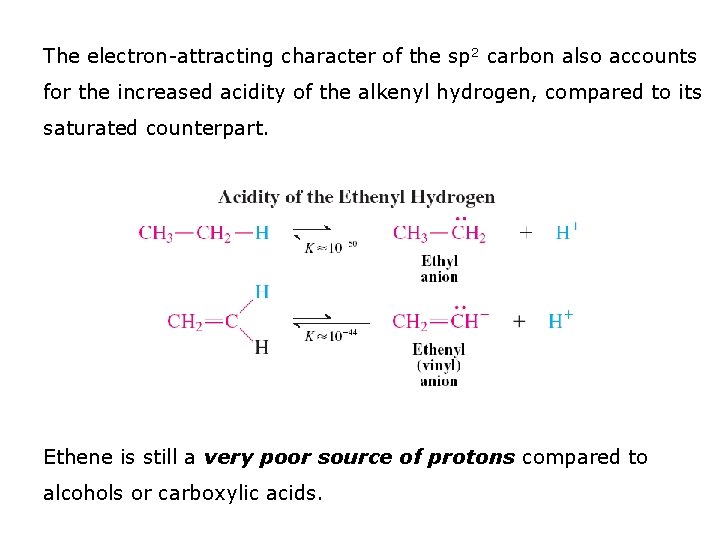
The electron-attracting character of the sp 2 carbon also accounts for the increased acidity of the alkenyl hydrogen, compared to its saturated counterpart. Ethene is still a very poor source of protons compared to alcohols or carboxylic acids.

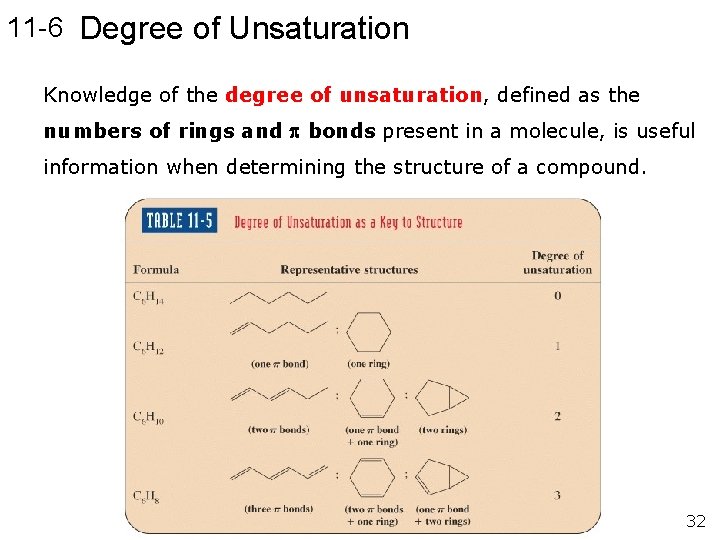
11 -6 Degree of Unsaturation Knowledge of the degree of unsaturation, defined as the numbers of rings and bonds present in a molecule, is useful information when determining the structure of a compound. 32

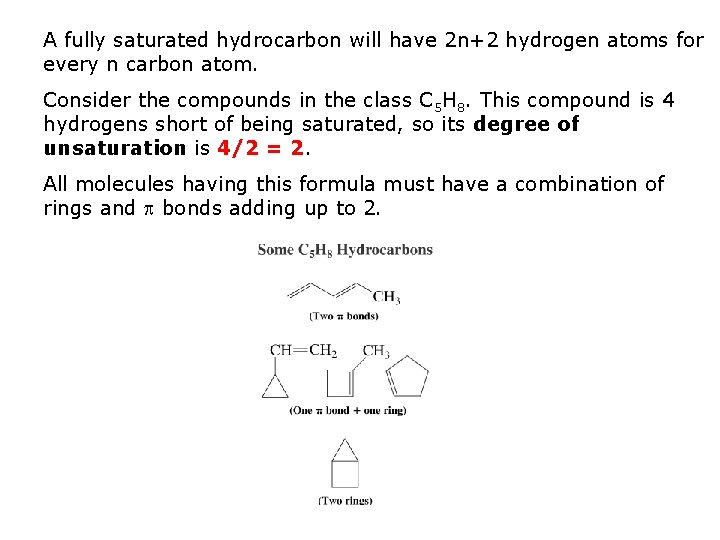
A fully saturated hydrocarbon will have 2 n+2 hydrogen atoms for every n carbon atom. Consider the compounds in the class C 5 H 8. This compound is 4 hydrogens short of being saturated, so its degree of unsaturation is 4/2 = 2. All molecules having this formula must have a combination of rings and bonds adding up to 2.

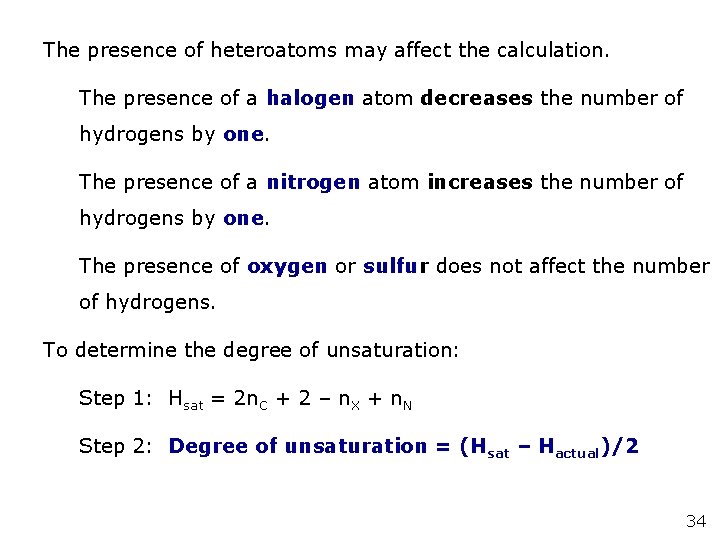
The presence of heteroatoms may affect the calculation. The presence of a halogen atom decreases the number of hydrogens by one. The presence of a nitrogen atom increases the number of hydrogens by one. The presence of oxygen or sulfur does not affect the number of hydrogens. To determine the degree of unsaturation: Step 1: Hsat = 2 n. C + 2 – n. X + n. N Step 2: Degree of unsaturation = (Hsat – Hactual)/2 34

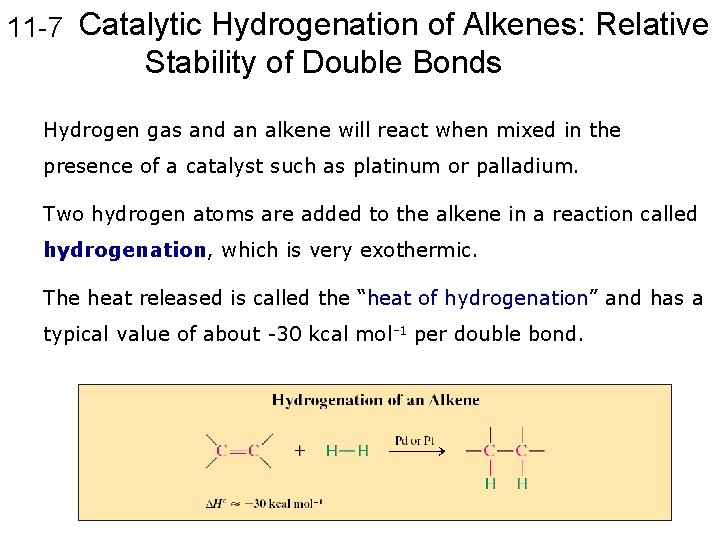
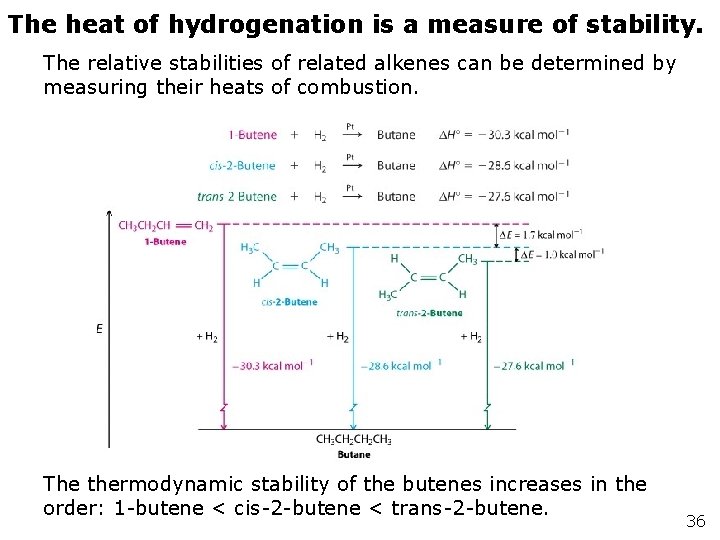
11 -7 Catalytic Hydrogenation of Alkenes: Relative Stability of Double Bonds Hydrogen gas and an alkene will react when mixed in the presence of a catalyst such as platinum or palladium. Two hydrogen atoms are added to the alkene in a reaction called hydrogenation, which is very exothermic. The heat released is called the “heat of hydrogenation” and has a typical value of about -30 kcal mol-1 per double bond.

The heat of hydrogenation is a measure of stability. The relative stabilities of related alkenes can be determined by measuring their heats of combustion. The thermodynamic stability of the butenes increases in the order: 1 -butene < cis-2 -butene < trans-2 -butene. 36

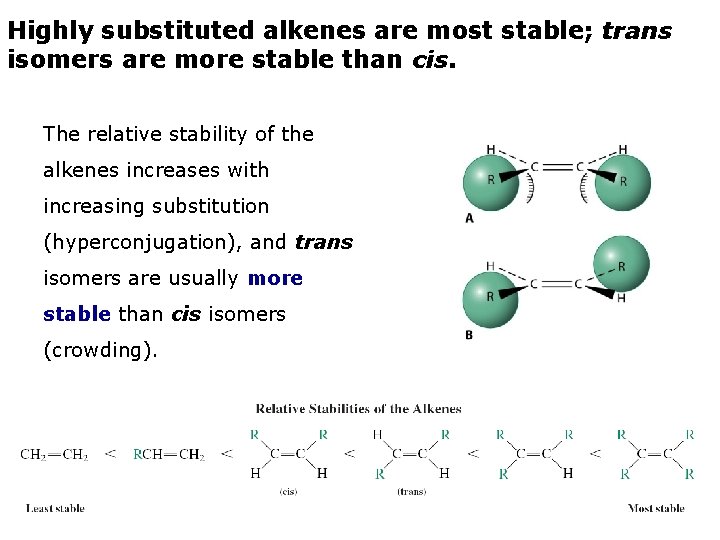
Highly substituted alkenes are most stable; trans isomers are more stable than cis. The relative stability of the alkenes increases with increasing substitution (hyperconjugation), and trans isomers are usually more stable than cis isomers (crowding).

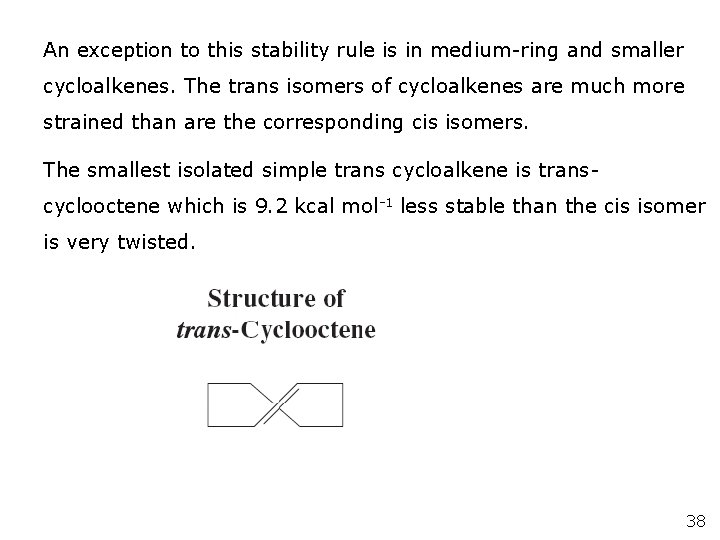
An exception to this stability rule is in medium-ring and smaller cycloalkenes. The trans isomers of cycloalkenes are much more strained than are the corresponding cis isomers. The smallest isolated simple trans cycloalkene is transcyclooctene which is 9. 2 kcal mol-1 less stable than the cis isomer is very twisted. 38

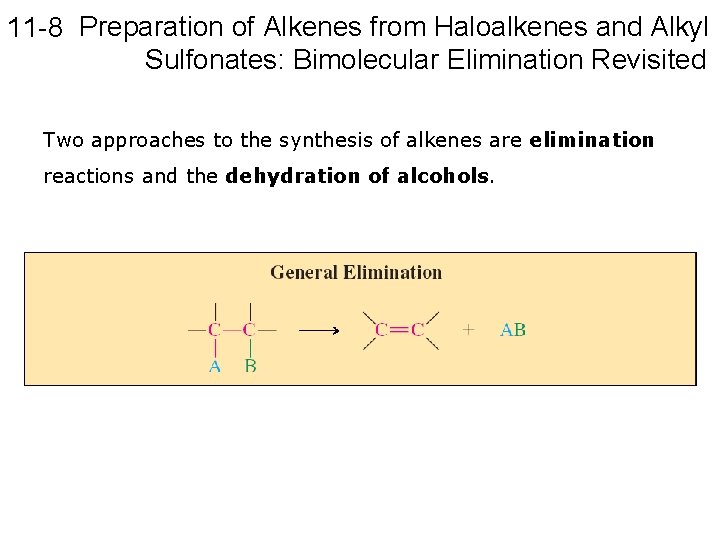
11 -8 Preparation of Alkenes from Haloalkenes and Alkyl Sulfonates: Bimolecular Elimination Revisited Two approaches to the synthesis of alkenes are elimination reactions and the dehydration of alcohols.

Regioselectivity in E 2 reactions depends on the base. Haloalkanes (or alkyl sulfonates) in the presence of strong base can undergo elimination of HX with the simultaneous formation of a C=C double bond. In the cases where the hydrogen atom can be removed from more than one carbon atom in the structure, the regioselectivity of the reaction can be controlled to a limited extent. 40

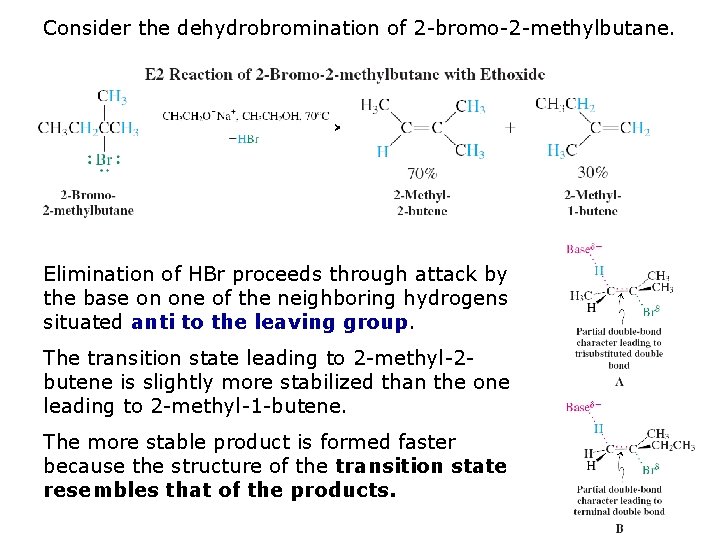
Consider the dehydrobromination of 2 -bromo-2 -methylbutane. Elimination of HBr proceeds through attack by the base on one of the neighboring hydrogens situated anti to the leaving group. The transition state leading to 2 -methyl-2 butene is slightly more stabilized than the one leading to 2 -methyl-1 -butene. The more stable product is formed faster because the structure of the transition state resembles that of the products.

Elimination reactions that lead to the more highly substituted alkene are said to follow the Saytzev rule. The double bond preferentially forms between the carbon that contained the leaving group and the most highly substituted adjacent carbon that bears a hydrogen. 42

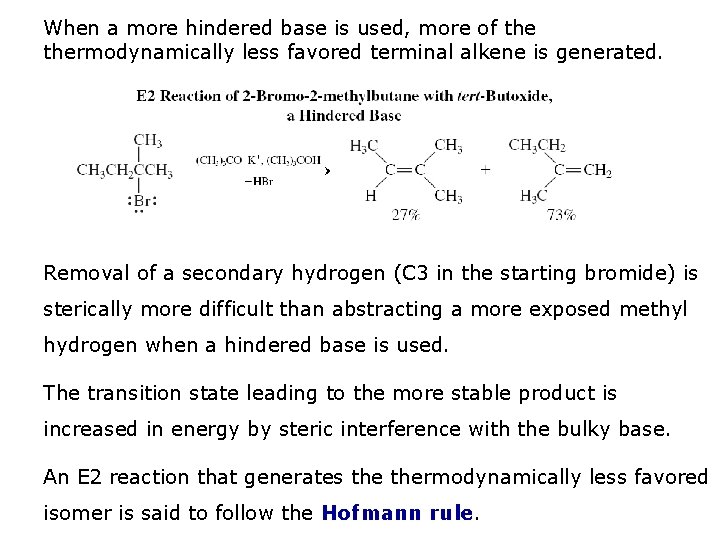
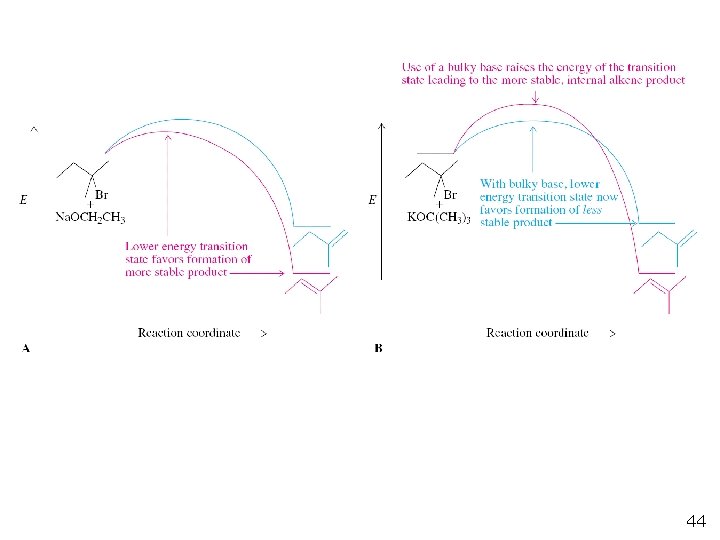
When a more hindered base is used, more of thermodynamically less favored terminal alkene is generated. Removal of a secondary hydrogen (C 3 in the starting bromide) is sterically more difficult than abstracting a more exposed methyl hydrogen when a hindered base is used. The transition state leading to the more stable product is increased in energy by steric interference with the bulky base. An E 2 reaction that generates thermodynamically less favored isomer is said to follow the Hofmann rule.

44

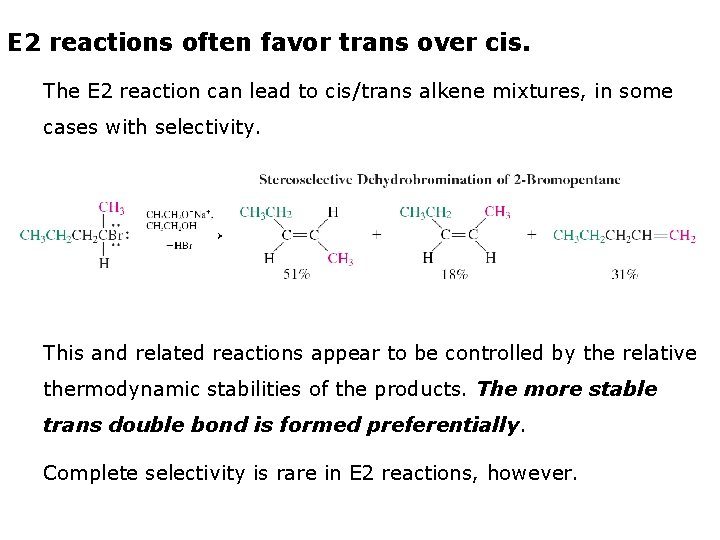
E 2 reactions often favor trans over cis. The E 2 reaction can lead to cis/trans alkene mixtures, in some cases with selectivity. This and related reactions appear to be controlled by the relative thermodynamic stabilities of the products. The more stable trans double bond is formed preferentially. Complete selectivity is rare in E 2 reactions, however.

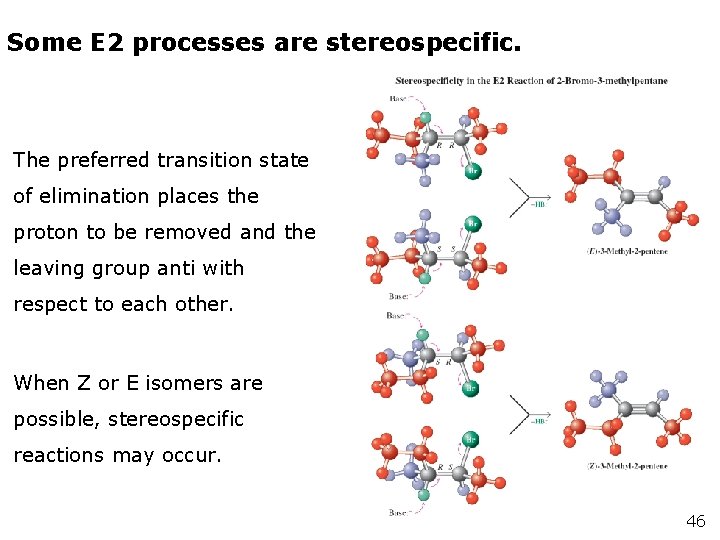
Some E 2 processes are stereospecific. The preferred transition state of elimination places the proton to be removed and the leaving group anti with respect to each other. When Z or E isomers are possible, stereospecific reactions may occur. 46

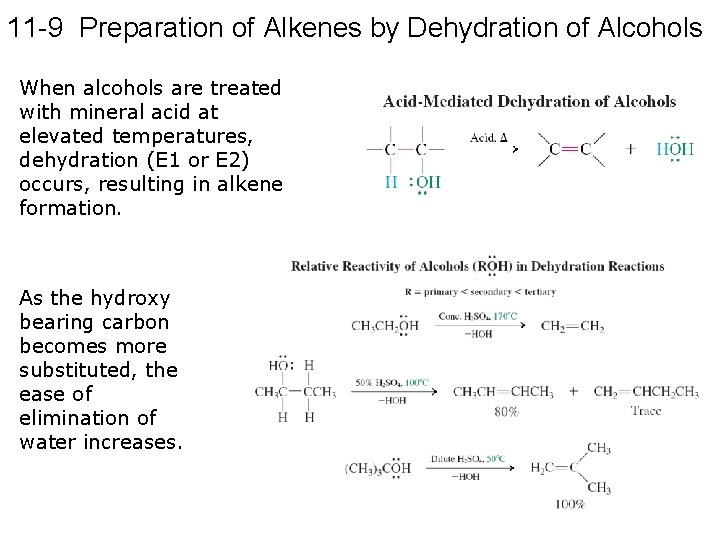
11 -9 Preparation of Alkenes by Dehydration of Alcohols When alcohols are treated with mineral acid at elevated temperatures, dehydration (E 1 or E 2) occurs, resulting in alkene formation. As the hydroxy bearing carbon becomes more substituted, the ease of elimination of water increases.

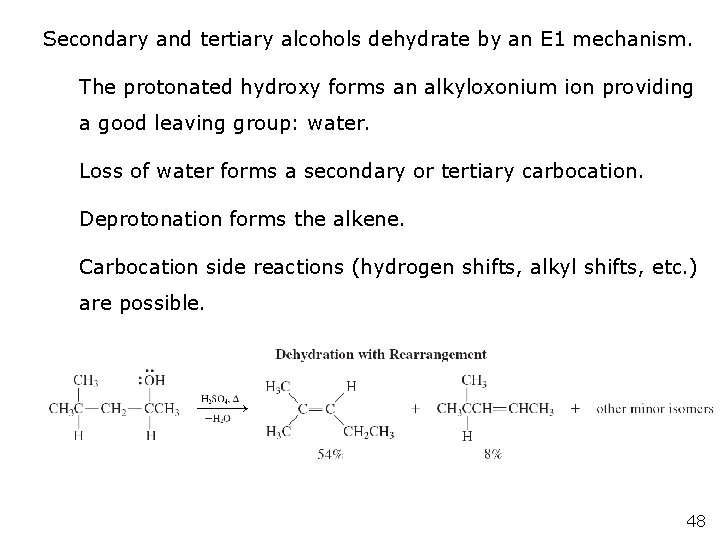
Secondary and tertiary alcohols dehydrate by an E 1 mechanism. The protonated hydroxy forms an alkyloxonium ion providing a good leaving group: water. Loss of water forms a secondary or tertiary carbocation. Deprotonation forms the alkene. Carbocation side reactions (hydrogen shifts, alkyl shifts, etc. ) are possible. 48

Thermodynamically most stable alkene or alkene mixture usually results from unimolecular dehydration in the presence of acid. Whenever possible, the most highly substituted system is generated. Trans-substituted alkenes predominate if there is a choice. Treatment of primary alcohols with mineral acids at high temperatures also leads to alkenes. The reaction of propanol yields propene. The reaction proceeds by protonation of the alcohol, followed by attack by HSO 4 - or another alcohol molecule (E 2 reaction) to remove a proton from one carbon atom and water from the other.

11 Important Concepts 1. Alkenes – Unsaturated molecules • • • IUPAC names are derived from the longest chain containing the double bond as the stem. Double bond isomers: terminal, internal, cis, and trans Tri- and tetra-substituted alkenes are named according to the E, Z system. 2. Double Bond – Consists of a bond • • • bond: overlap of two sp 2 hybrid lobes on carbon bond: overlap of two remaining p orbitals bond E (~65 kcal/mol); bond E (~108 kcal/mol) 50

11 Important Concepts 3. Alkene Properties – • • • Flat, sp 2 hybridization Dipoles possible Alkenyl hydrogen is relatively acidic. 4. NMR – • • • Alkenyl hydrogens and carbons appear at low field: 1 H (δ = 4. 6 - 5. 7 ppm); 13 C (δ = 100 -140 ppm) Jtrans > Jcis; jgeminal very small; Jallylic variable, small. 5. IR – Measures vibration excitation • • • 1 -10 kcal/mol (2. 5 -16. 7 μm; 600 -4000 cm-1) Characteristic peaks for stretching, bending and other vibrational modes Fingerprint region (<1500 cm-1)

11 Important Concepts 6. Alkane IR – • • • C-H Stretching: 2840 to 3000 cm-1 C=C Stretching: 1620 to 1680 cm-1 Alkenyl C-H Stretching: ~3100 cm-1 Bending Modes: below 1500 cm-1 Alcohols: Broad O-H stretch: between 3200 and 3650 cm-1 7. Degree of Unsaturation – Number of rings + number of bonds: • Degree of unsaturation = (Hsat – Hactual)/2 • Hsat = 2 n. C + 2 – NX + NN (disregard oxygen and sulfur) 52

11 Important Concepts 8. Heats of Hydrogenation – indicate relative stability of isomeric alkenes. • Stability decreases with decreasing substitution. • Trans isomers are more stable than cis. 9. Eliminations of Haloalkanes (and other alkyl derivatives) – • • • Follow the Sayzex rule (non-bulky base, internal alkene formation) or the Hofmann rule (bulky base, terminal alkene formation). Trans alkene products predominate over cis. Elimination is stereospecific (dictated by the anti transition state).

11 Important Concepts 10. Dehydration of Alcohols – Dehydration in the presence of strong acid results in a mixture of products (major constituent is the most stable alkene). 54