Change of Phase Chapter 17 Evaporation Evaporation A






























- Slides: 33

Change of Phase Chapter 17

Evaporation

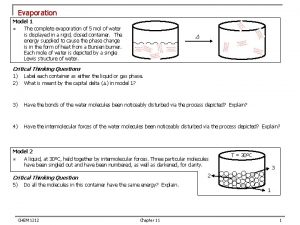
Evaporation �A change of phase from liquid to gas that occurs at the surface of a liquid.

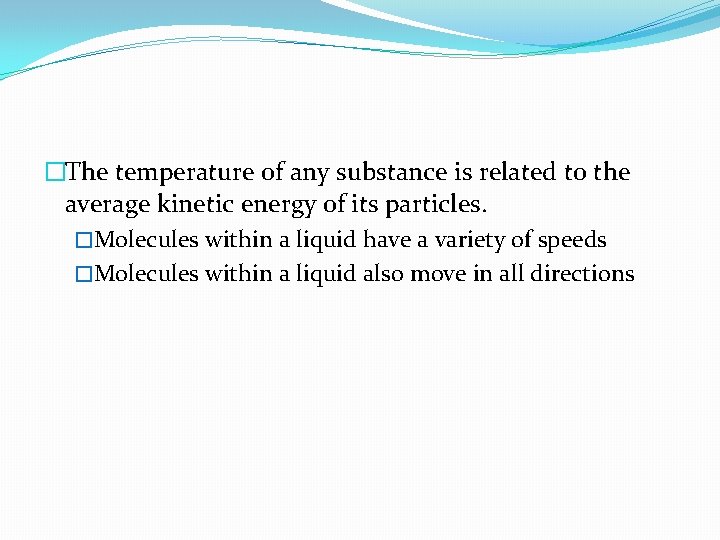
�The temperature of any substance is related to the average kinetic energy of its particles. �Molecules within a liquid have a variety of speeds �Molecules within a liquid also move in all directions

�Molecules at the surface gain kinetic energy while others lose kinetic energy �Molecules gain KE at the surface by bumping into each other

�Increased KE of molecules bumped hard enough to break free from the liquid comes from the molecules remaining in the liquid. �When molecules bumped into one another �Some gain KE �Others lose KE � The average KE of the molecules remaining in the liquid is lowered � Evaporation is a cooling process

�The fast molecules that break free from the surface are slowed as they fly away due to their attraction to the surface. �So although water is cooled by evaporation, the air above is not correspondingly warmed by the process.

Condensation

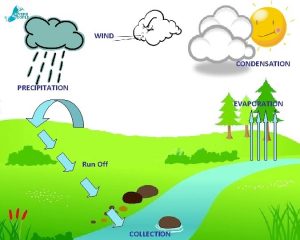
Condensation �The changing of a gas to a liquid �This is the opposite of evaporation

�When gas molecules near the surface of a liquid are attracted to the liquid, they strike the surface with increased KE and become apart of the liquid �Excess KE is shared with the liquid �This increases the liquids temperature �Condensation is a warming process

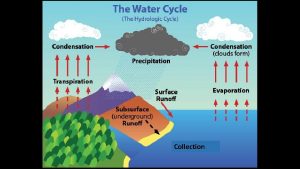
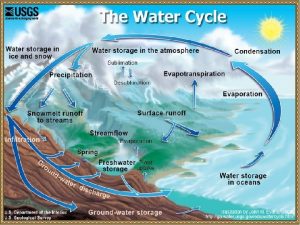
Condensation in the Atmosphere �There is always some water vapor in the air �A measure of the amount of this water vaopr is called humidity

Fog and Clouds �Warm air rises. �As it rises it expands. �As it expands it chills �As the air chills water-vapor molecules are slowed. �Lower-speed molecular collisions result in water molecules sticking together. �If there are larger and slower-moving particles or ions present, water vapor condenses upon these particles, and with sufficient buildup we have a cloud

Boiling

�Evaporation can take place beneath the surface of a liquid, forming bubbles of vapor that are buoyed to the surface, where they escape. �This change of phase throughout a liquid rather than only at the surface is called boiling.

�Bubbles in the liquid can form only when the pressure of the vapor within the bubble is great enough to resist the pressure of the surrounding liquid �So bubbles do not form until the boiling point is reached

�If the pressure is increased, the molecules in the vapor must move fast to exert enough pressure to keep the bubble form collapsing. �Extra pressure can be provided either by going deeper below the surface of the liquid �Like in geysers �Or by increasing the air pressure above the liquids surface �Which is how a pressure cooker works.

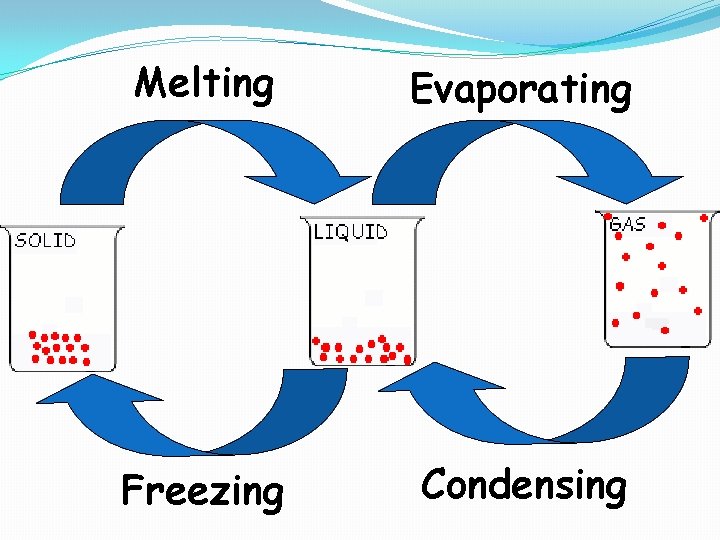
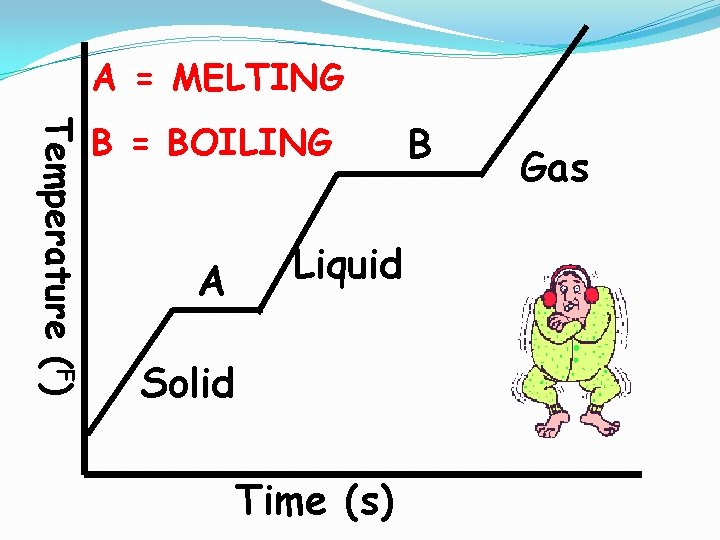
Melting Evaporating Freezing Condensing

A = MELTING Temperature (F) B = BOILING A Liquid Solid Time (s) B Gas

Melting & Freezing

�Suppose that you are holding hands with someone and both of you start jumping. �What happens as you start to jump around randomly?

�Something like this happens to the molecules of a solid when it is heated. �As heat is absorbed, the molecules vibrate more and more violently. �If enough heat is absorbed, the attractive forces between the molecules will no longer be able to hold them together �The solid melts

�Freezing is the opposite of this process �Energy is withdrawn from a liquid, molecular motion diminishes until finally the molecules, on the average are moving slowly enough so that the attractive forces between them are able to cause cohesion. �The molecules then vibrate about a fixed position and form a solid

�What would happen if you were to add salt or sugar to water? �Would it freeze faster, slower, the same? �Why?

Regelation

�Melting under pressure and freezing again when the pressure is reduced �The ability to do this is one of the properties of water that distinguishes if from other materials �Can you think of any examples?

Energy and Changes of Phase

�If we continually add heat to a sold or liquid, the solid or liquid will eventually change phase �Energy input is required for both liquefaction of the solid and vaporization of a liquid �Energy must be extracted to reverse the process

�A refrigerator is a heat pump that “pumps” heat from a cold environment to a warm one. �This is accomplished by a liquid of low boiling point, the refrigerant, which is pumped into the cooling unit, where it turns into a gas

�Heat pumps of various designs are increasingly being utilized to heat and cool homes �What these heat pumps have in common with one another is that they function like a standard refrigerator.

�An air conditioner is a heat pump in reverse. �Employing the same principles, it simply pumps heat energy from inside the home to the outside

Quick Check �In order to become a liquid a solid must _______ energy. �In order to become a liquid a gas must ____ energy.

Heat of Fusion �Either the energy needed to separate molecules from the solid phase or the energy released when bonds form in a liquid that change it to the solid phase

Heat of Vaporization �Either the energy required to separate molecules from the liquid phase or the energy released when gas condenses to the liquid phase
 Chapter 23 change of phase
Chapter 23 change of phase Chapter 7 heat transfer and change of phase
Chapter 7 heat transfer and change of phase Conceptual physics chapter 17 change of phase answers
Conceptual physics chapter 17 change of phase answers Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Tswett pronunciation
Tswett pronunciation Mobile phase and stationary phase
Mobile phase and stationary phase Chromatography mobile phase and stationary phase
Chromatography mobile phase and stationary phase Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Phase to phase voltage
Phase to phase voltage Which detector used in hplc
Which detector used in hplc Phase to phase voltage
Phase to phase voltage Csce 441
Csce 441 Phase change formula
Phase change formula Phase change formula
Phase change formula Phase change formula
Phase change formula Phase changes in matter
Phase changes in matter All states of matter
All states of matter Examples of phase change
Examples of phase change Is vaporization endothermic or exothermic
Is vaporization endothermic or exothermic Phase change worksheet
Phase change worksheet Solid liquid gas
Solid liquid gas States of matter concept map
States of matter concept map Phase change concept map
Phase change concept map Particles
Particles Phase change concept map
Phase change concept map Phase change foldable
Phase change foldable Phase change materials
Phase change materials Emersion circulator
Emersion circulator Phase change material
Phase change material What phase change is boiling water
What phase change is boiling water Phase change diagram endothermic exothermic
Phase change diagram endothermic exothermic Gas to plasma
Gas to plasma Phase change memory
Phase change memory Examples of physical changes
Examples of physical changes