Do Now Homework water cycle worksheet Phase Changes
















- Slides: 35

Do Now • Homework: water cycle worksheet

Phase Changes Day 1 What did one water molecule say to another water molecule about vapor? Don’t worry it’s just a phase he’ll cool down.

Standard/ Objective Standard: PS 3. 1 c The motion of particles helps to explain the phases/states of matter as well as changes from one phase to another. The phase in which matter exists depends on the attractive forces among its particles. Objective: SWBAT describe and list the changes that occur between the liquid and gas states.

Flow of the Day • Do Now 5 min • Notes 8 min • Guided practice 8 min • Independent practice 8 min • Journal 8 min • Exit Slip 5 min

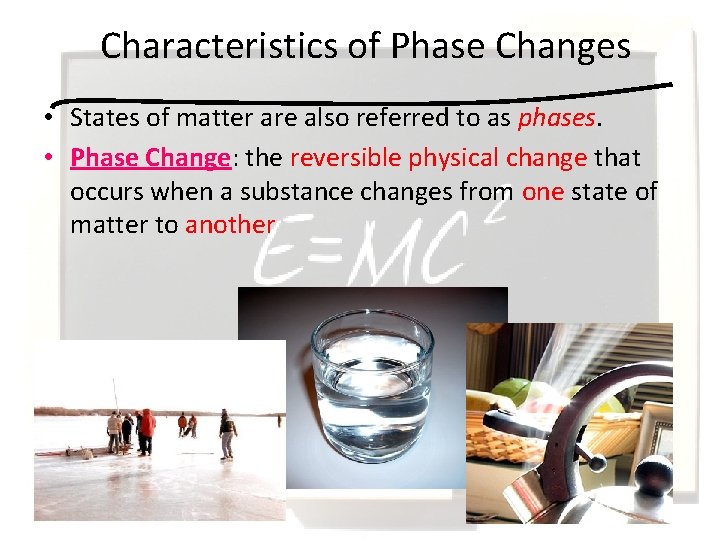
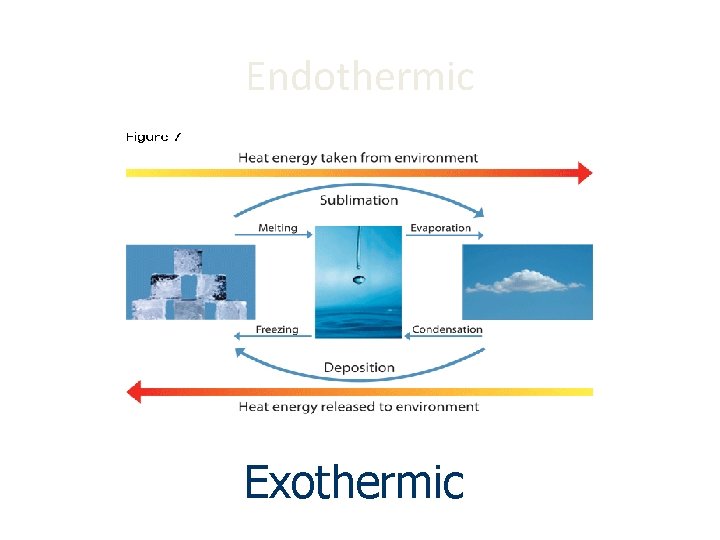
Characteristics of Phase Changes • States of matter are also referred to as phases. • Phase Change: the reversible physical change that occurs when a substance changes from one state of matter to another

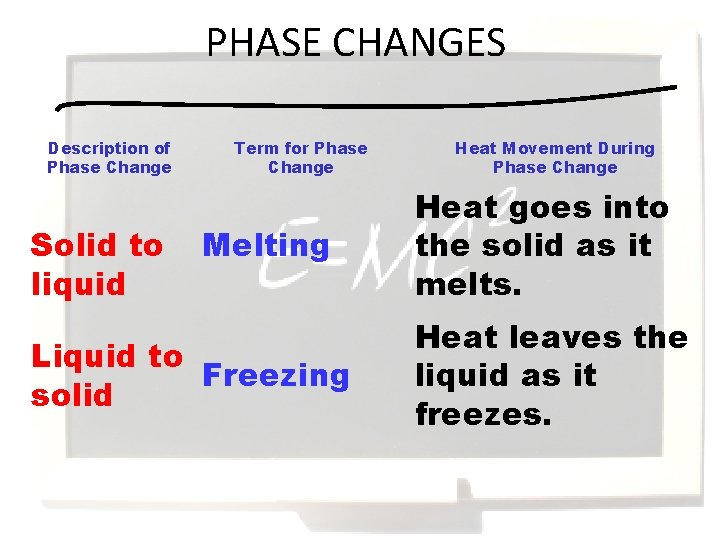
PHASE CHANGES Description of Phase Change Solid to liquid Term for Phase Change Melting Liquid to Freezing solid Heat Movement During Phase Change Heat goes into the solid as it melts. Heat leaves the liquid as it freezes.

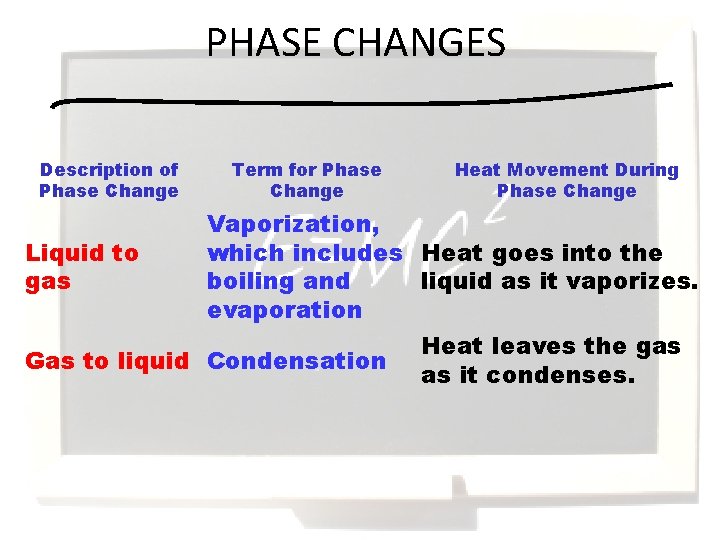
PHASE CHANGES Description of Phase Change Term for Phase Change Heat Movement During Phase Change Vaporization, which includes Heat goes into the Liquid to gas boiling and liquid as it vaporizes. evaporation Heat leaves the gas Gas to liquid Condensation as it condenses.

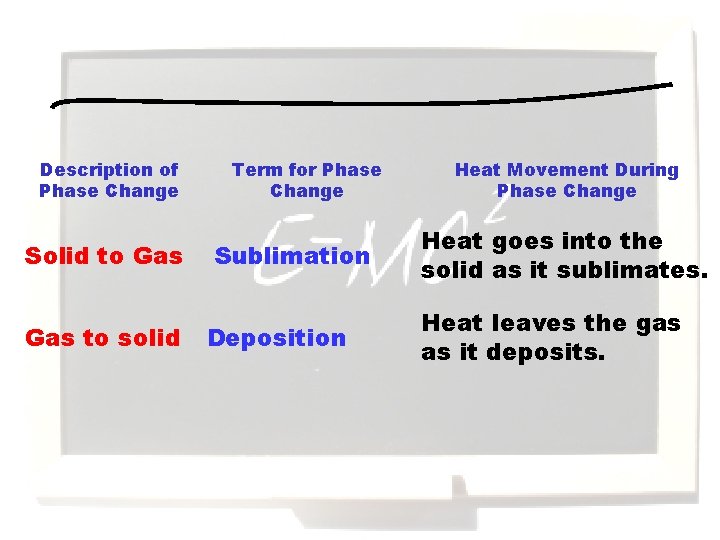
Description of Phase Change Solid to Gas to solid Term for Phase Change Sublimation Deposition Heat Movement During Phase Change Heat goes into the solid as it sublimates. Heat leaves the gas as it deposits.

Guided Practice Fill out the diagram on your worksheet using the notes we just took. We will go over the answers as a class when you have finished.

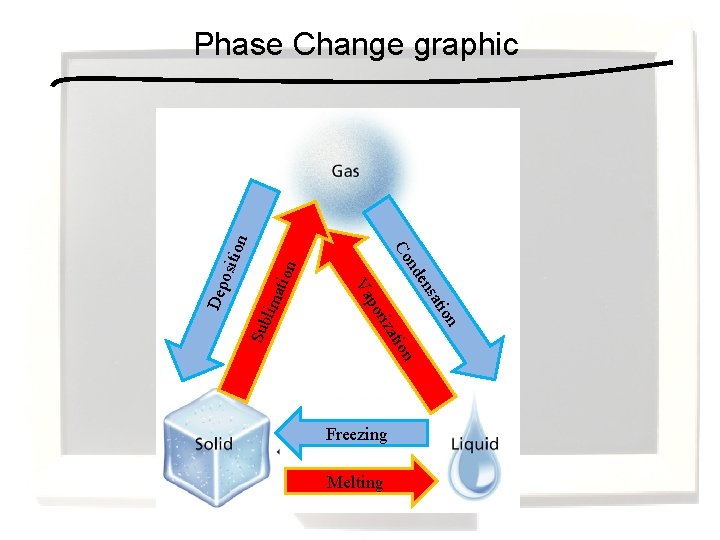
Freezing Melting on tion ima i sat on ati riz po Sub l en Va Dep osit nd Co ion Phase Change graphic

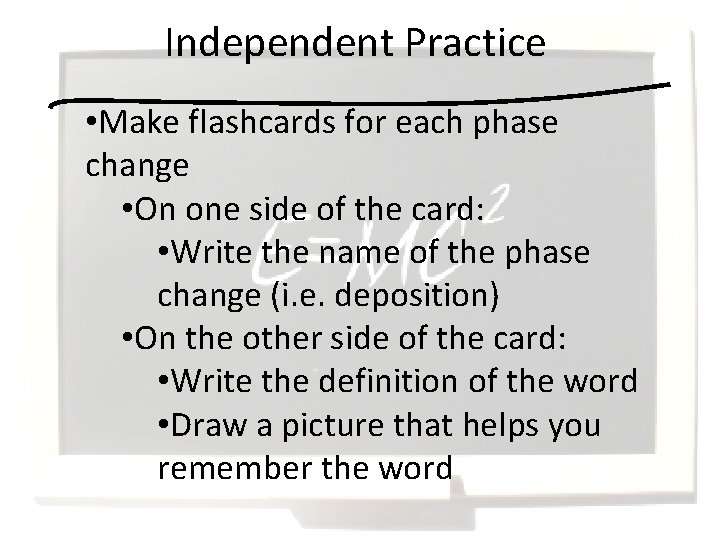
Independent Practice • Make flashcards for each phase change • On one side of the card: • Write the name of the phase change (i. e. deposition) • On the other side of the card: • Write the definition of the word • Draw a picture that helps you remember the word

Journal • What did you learn today? Why is it important?

Exit Slip Hat • What is the phase change from solid to liquid called? • What is the phase change from a liquid to a solid called?

Do Now • Homework: States of matter worksheet

Phase Changes Day 2 What did one water molecule say to another water molecule about vapor? Don’t worry it’s just a phase he’ll cool down.

Standard/ Objective Standard: PS 3. 1 c The motion of particles helps to explain the phases/states of matter as well as changes from one phase to another. The phase in which matter exists depends on the attractive forces among its particles. Objective: SWBAT explain that the motion of particles is the reason that matter can change from one phase to another

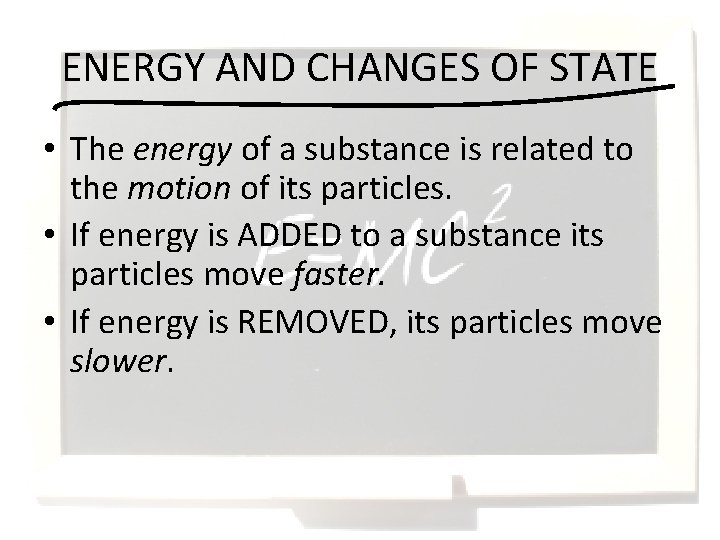
ENERGY AND CHANGES OF STATE • The energy of a substance is related to the motion of its particles. • If energy is ADDED to a substance its particles move faster. • If energy is REMOVED, its particles move slower.

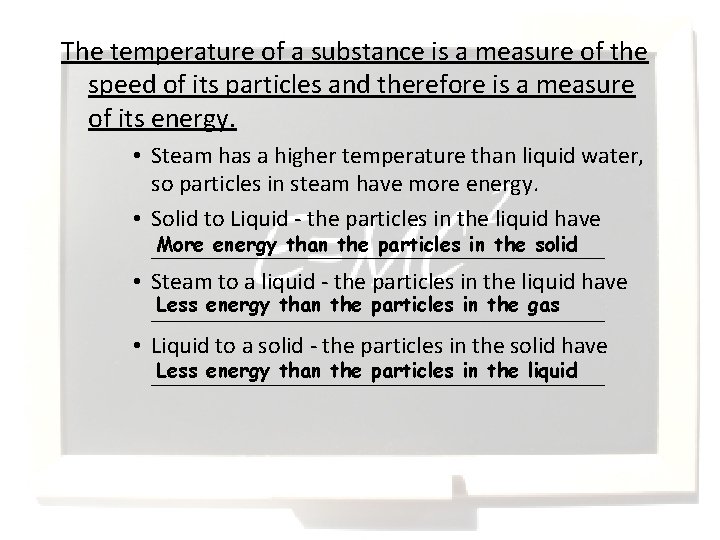
The temperature of a substance is a measure of the speed of its particles and therefore is a measure of its energy. • Steam has a higher temperature than liquid water, so particles in steam have more energy. • Solid to Liquid - the particles in the liquid have More energy than the particles in the solid ___________________ • Steam to a liquid - the particles in the liquid have Less energy than the particles in the gas ___________________ • Liquid to a solid - the particles in the solid have Less energy than the particles in the liquid ___________________

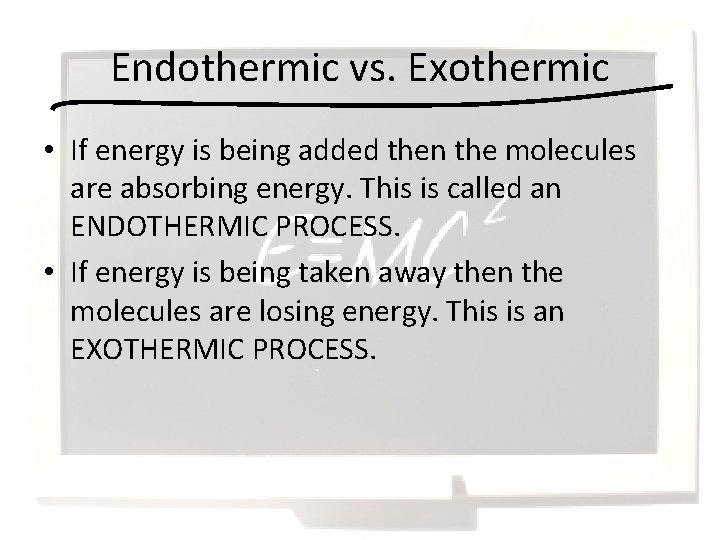
Endothermic vs. Exothermic • If energy is being added then the molecules are absorbing energy. This is called an ENDOTHERMIC PROCESS. • If energy is being taken away then the molecules are losing energy. This is an EXOTHERMIC PROCESS.

Which are endothermic and which are exothemic? • • • Liquid to a solid - freezing Gas to a liquid -condensation Solid to a liquid - melting Solid to a gas – sublimation Liquid to a gas - vaporization

Endothermic Exothermic

Journal Why is energy important in phase changes? Use the words for endothermic and exothermic in your answer.

Exit Slip Standard

Do Now • Make a list and draw a picture of the six simple machines! • Homework: • Oobleck packet: Writing about physical properties. • If you don’t have the packet, this is the assignment: Describe what your favorite food is to someone without telling them what it is (use words that have to do with the five senses-sight, touch, hearing, smell and taste). • Study for Quiz Friday

Phase Changes Day 3 What did one water molecule say to another water molecule about vapor? Don’t worry it’s just a phase he’ll cool down.

Standard/ Objective Standard: PS 4. 2 c - During a phase change, heat energy is absorbed or released. Energy is absorbed when a solid changes to a liquid and when a liquid changes to a gas. Energy is released when a gas changes to a liquid and when a liquid changes to a solid. Objective: SWBAT describe the role of thermal energy on phase change.

Flow of the Day • • • Do Now 5 min Intro to new material 5 min Guided Practice 15 min Exit slip 5 min Journal 8 min

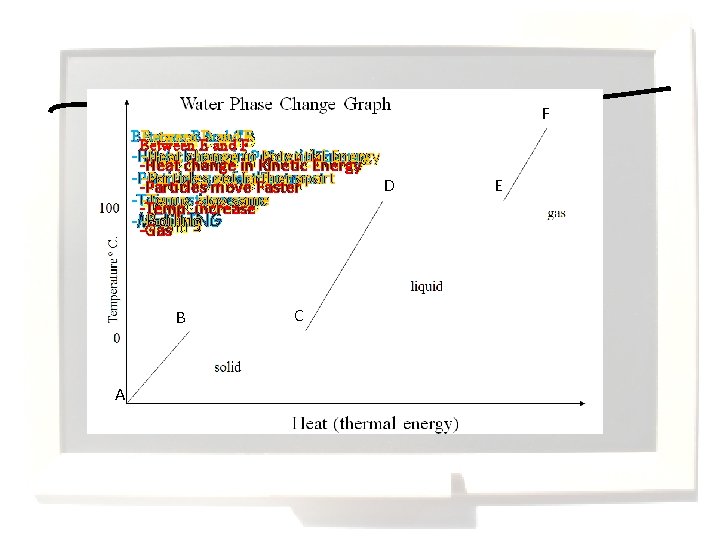
Average Kinetic Energy = Temperature is a measurement of the average kinetic energy of the particles within a substance. Meaning that temperature measures movement of particles. Temperature is proportional to the average kinetic energy of the molecules of a substance. That means if you double the Kelvin temperature of a substance, you double the average kinetic energy of its molecules. When the average kinetic energy of the molecules goes up (a rise in temperature), the average speed of the molecules increases. A change in average kinetic energy is not directly proportional to a change in average speed.

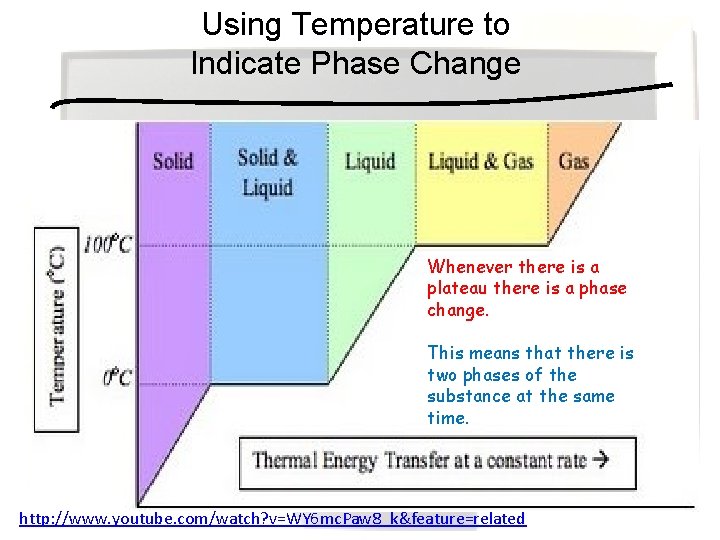
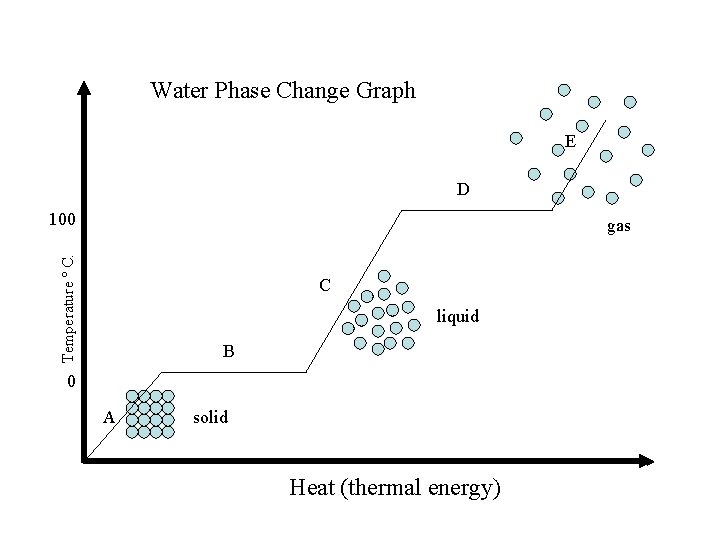
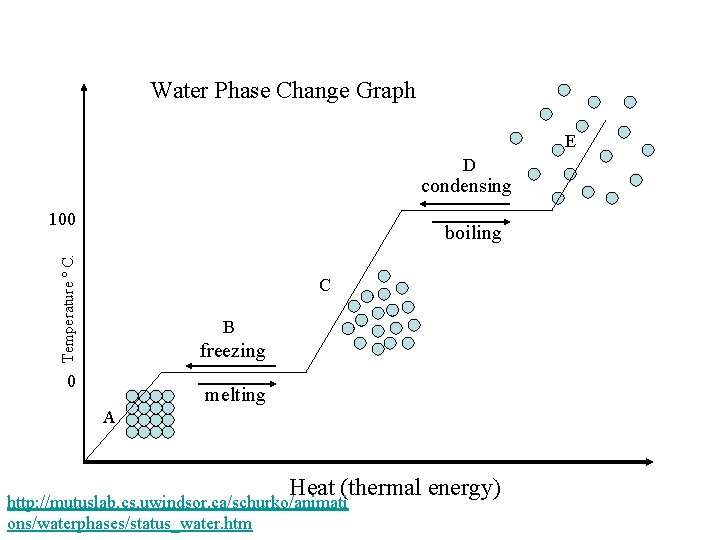
Using Temperature to Indicate Phase Change Whenever there is a plateau there is a phase change. This means that there is two phases of the substance at the same time. http: //www. youtube. com/watch? v=WY 6 mc. Paw 8_k&feature=related

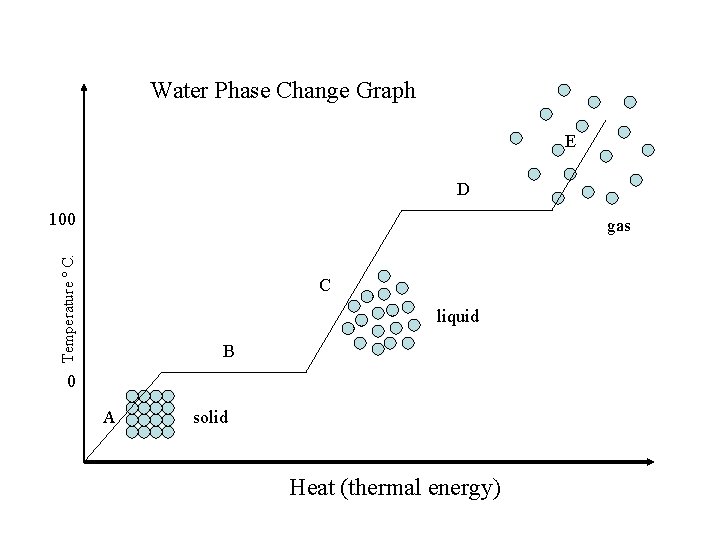
Water Phase Change Graph E D 100 Temperature º C. gas C liquid B 0 A solid Heat (thermal energy)

Water Phase Change Graph E D 100 Temperature º C. gas C liquid B 0 A solid Heat (thermal energy)

Water Phase Change Graph E D condensing 100 Temperature º C. boiling C B freezing 0 melting A Heat (thermal energy) http: //mutuslab. cs. uwindsor. ca/schurko/animati ons/waterphases/status_water. htm

F Between and A B Between. BED Cand and. CFE D Between and -Heat change in Potential Energy -Heat change in Kinetic Energy -Particles get farther apart -Particles move Faster D -Particles move Faster -Temp. stays same -Temp. stay same -Temp. increase -MELTING -Boiling -Solid -liquid -Gas B A C E

Journal Describe how the phases change in the water cycle.

Exit Slip Standard: PS 4. 2 c - During a phase change, heat energy is absorbed or released. Energy is absorbed when a solid changes to a liquid and when a liquid changes to a gas. Energy is released when a gas changes to a liquid and when a liquid changes to a solid. Objective: SWBAT describe the role of thermal energy on phase change.