Evaporation Evaporation the process in which water changes


















- Slides: 21

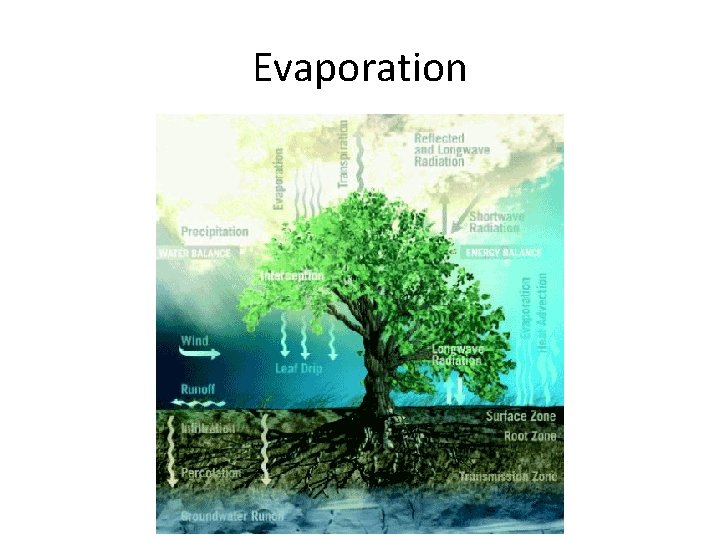
Evaporation

Evaporation • the process in which water changes phase from liquid to vapor and is transported from the Earth’s surface to the atmosphere • We will use the following distinctions – transpiration (T): through plants – evaporation (E): from soil, the exterior surfaces of plants, or surface water bodies – evapotranspiration (ET): ET = E + T

Necessary conditions for evaporation • A supply of heat – latent heat of vaporization: the energy input required to overcome the molecular forces of attraction between water molecules in liquid form – 2. 5 x 106 J kg-1 at 15 C • the joule (J) is the SI unit of energy (kg m 2 s-2) • 4. 18 J = 1 calorie – heat can come from external sources or can be withdrawn from the body undergoing evaporation

Necessary conditions for evaporation • A vapor pressure gradient – vapor pressure of the atmosphere < vapor pressure of the evaporating surface – this gradient drives transport of water by diffusion – transport by convection (bulk air flow) is also important

Necessary conditions for evaporation • A supply of water – sufficient water transport from or through the interior of the body to the site of evaporation • Thus evaporation can be limited by either – the evaporative demand: the supply of heat and the transport of vapor away from the surface – the soil: its ability to transport water to the surface

Evaporation from a water table • the greater the capillary rise the greater the potential for evaporation from the water table • shallow water tables are major contributors to the problem of soil salinization




Steady evaporation from a water table • assume evaporation is occurring but no change in soil water content • steady-state flow • apply Buckingham-Darcy Law

Reading assignment • Evaporation, p. 337 -351

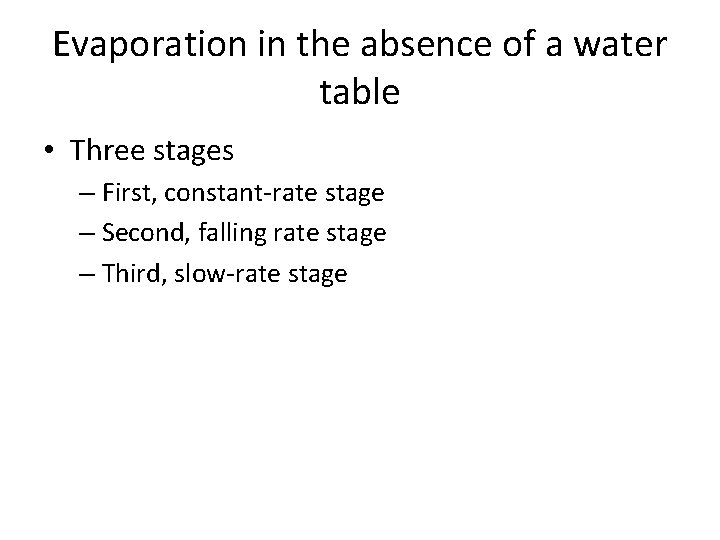
Evaporation in the absence of a water table • Three stages – First, constant-rate stage – Second, falling rate stage – Third, slow-rate stage







Reducing evaporation • During first or constant rate stage – Reduce the evaporative demand – Maintain soil cover – Reduce irrigation frequency • During second or falling rate stage – Decrease the hydraulic conductivity rapidly – “Dust mulch” was advocated in the early 1900’s • These management practices usually involve tradeoffs and should be carefully evalutated.

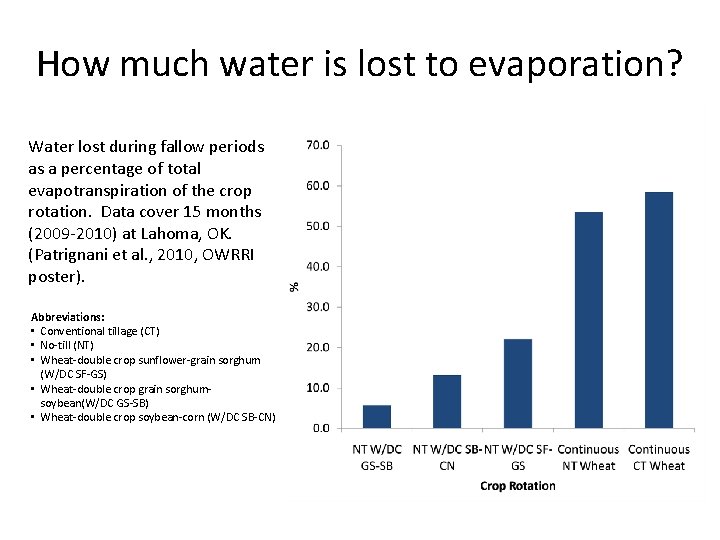
How much water is lost to evaporation? Water lost during fallow periods as a percentage of total evapotranspiration of the crop rotation. Data cover 15 months (2009 -2010) at Lahoma, OK. (Patrignani et al. , 2010, OWRRI poster). Abbreviations: • Conventional tillage (CT) • No-till (NT) • Wheat-double crop sunflower-grain sorghum (W/DC SF-GS) • Wheat-double crop grain sorghumsoybean(W/DC GS-SB) • Wheat-double crop soybean-corn (W/DC SB-CN)

Reading assignment • Plant uptake of soil moisture, p. 365 -378