Change of Phase Chapter 23 Evaporation Evaporation a








- Slides: 13

Change of Phase Chapter 23

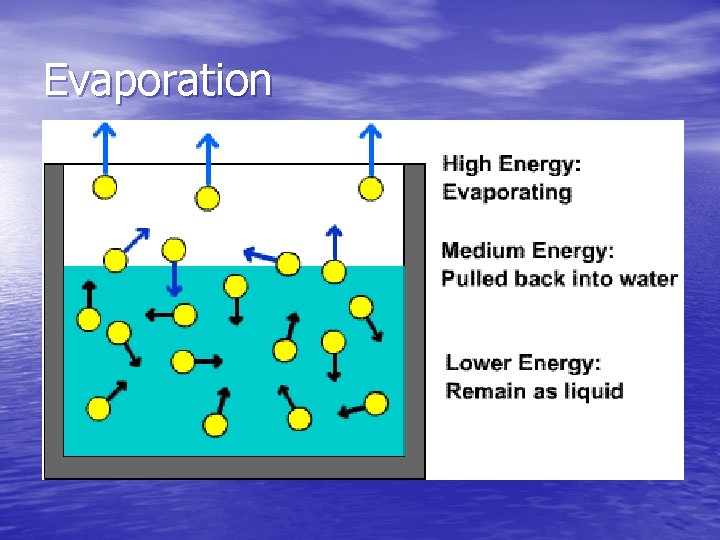
Evaporation • Evaporation – a change of phase from liquid to gas that takes place at the surface of a liquid • The molecules at the surface of a liquid may gain enough kinetic energy by being bumped by other molecules to break free of the liquid, comprising a vapor • The average kinetic energy of the molecules left behind is lowered, therefore evaporation is a cooling process

Evaporation

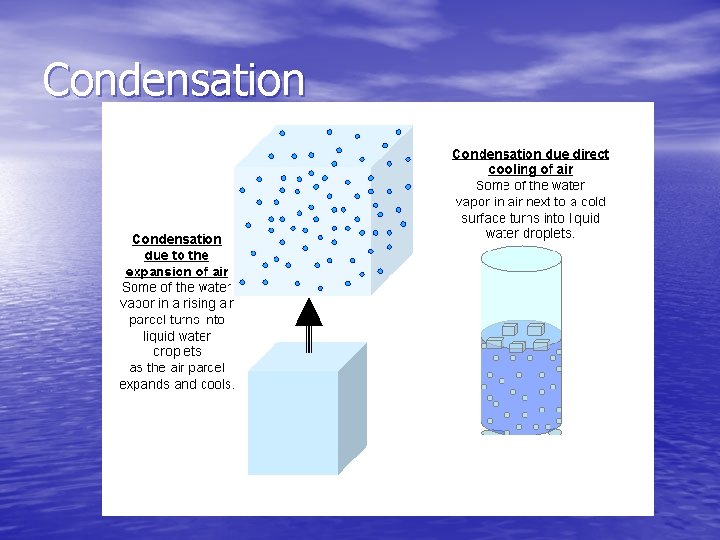
Condensation • Condensation – the changing of phase from a gas to • • • a liquid Vapor molecules can run into slower-moving particles of a cool surface and slow themselves down enough to reform as a liquid The air always contains some water vapor Saturated – at any given temperature, there is a limit to the amount of water vapor that can be in the air Relative Humidity – indicates how much water vapor is in the air, compared with the limit for that temperature At a relative humidity of 100%, the air is saturated Fog is formed when water vapors stick together as they cool, moist air moves in close to the ground and water vapor condenses out to form a cloud

Condensation

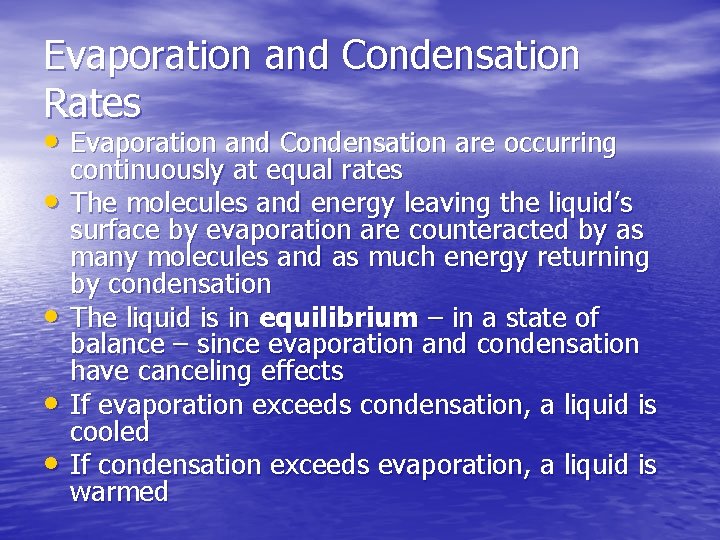
Evaporation and Condensation Rates • Evaporation and Condensation are occurring • • continuously at equal rates The molecules and energy leaving the liquid’s surface by evaporation are counteracted by as many molecules and as much energy returning by condensation The liquid is in equilibrium – in a state of balance – since evaporation and condensation have canceling effects If evaporation exceeds condensation, a liquid is cooled If condensation exceeds evaporation, a liquid is warmed

Boiling • Boiling – change of phase which occurs • • • beneath the surface from liquid to gas The pressure of the vapor within the bubbles of a boiling liquid must be great enough to resist the pressure of the surrounding water As atmospheric pressure is increased, the molecules in a vapor are required to move faster to exert increased pressure within the bubble, thereby increasing the boiling point of a liquid Boiling, like evaporation, is a cooling process

Freezing • Freezing – the change in phase from a liquid to a solid • When energy is extracted from a liquid, it freezes • If sugar or salt is dissolved in water, the freezing temperature is lowered; these molecules get in the way of the formation of ice crystals • The ocean, with its high salinity, rarely freezes, even on the top

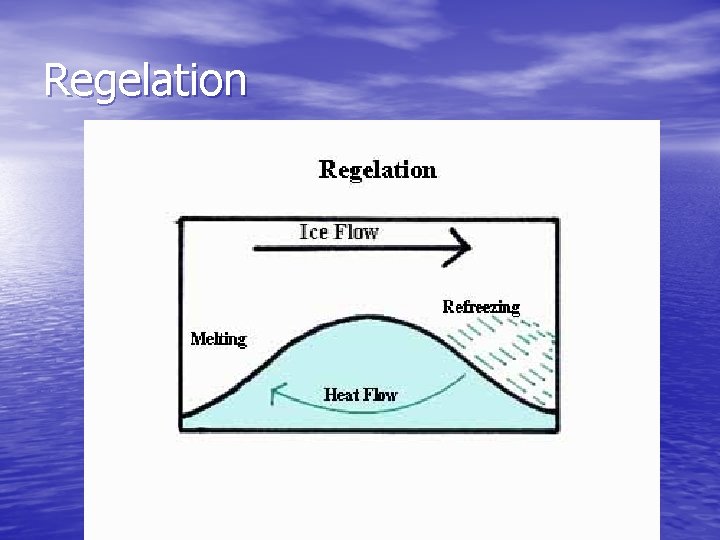
Regelation • Regelation – the phenomenon of melting under pressure and freezing again when the pressure is reduced (specific to water) • This is do to the very open structure of ice crystals, the application of pressure lowers the melting point

Regelation

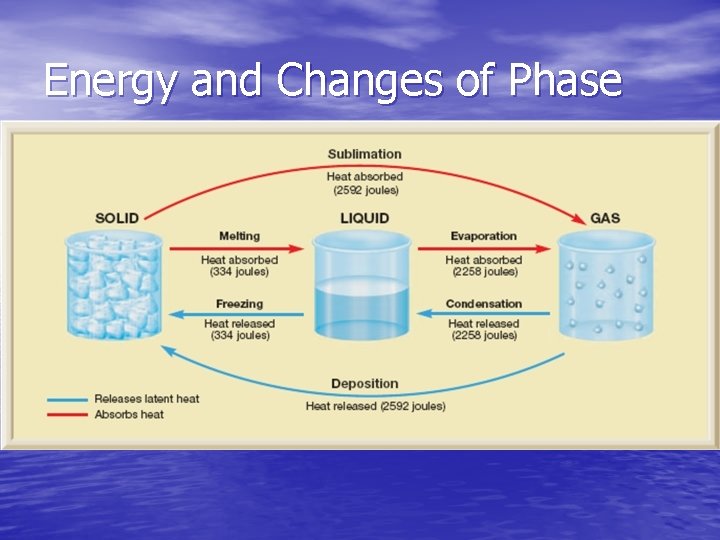
Energy and Changes of Phase • Energy must be put into a substance to change • • • from a solid all the way to a gas, and energy must be extracted to change a substances phase from a gas to a solid The phase change sequence is reversible! You can boil an ice cube, and then turn it back into an ice cube While a substance changes phase, its temperature does not change Much more energy is given off when water vapor condenses than when an equal mass of water freezes

Energy and Changes of Phase

Assignment • Read Chapter 23 (pg. 339 -350) • Do Chapter 23 #26 -47 (pg. 352 -353)
 Chapter 23 change of phase
Chapter 23 change of phase Chapter 7 heat transfer and change of phase
Chapter 7 heat transfer and change of phase Conceptual physics chapter 17 change of phase answers
Conceptual physics chapter 17 change of phase answers Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Hplc reverse phase vs normal phase
Hplc reverse phase vs normal phase Mobile phase and stationary phase
Mobile phase and stationary phase Mobile phase vs stationary phase
Mobile phase vs stationary phase Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Line current and phase current
Line current and phase current Adsorption chromatography
Adsorption chromatography In a triangle connected source feeding a y connected load
In a triangle connected source feeding a y connected load Csce 441
Csce 441 Phase change formula
Phase change formula Phase change formula
Phase change formula