Chapter 17 Evaporation Evaporation Occurs when high kinetic








































- Slides: 77

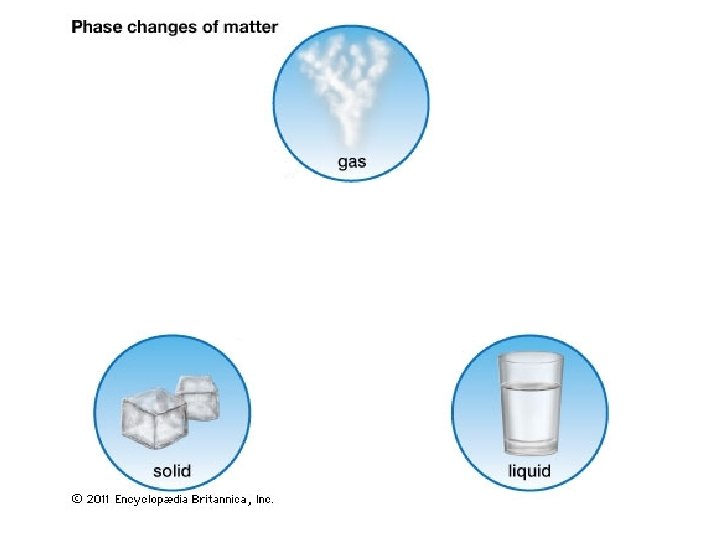
Chapter 17

Evaporation

Evaporation • Occurs when high kinetic energy particles at the surface of a liquid break free from the attractive forces of the neighboring particles and become vapor.

Vapor • A vapor is the gaseous state of a substance that is liquid or solid under ordinary conditions.

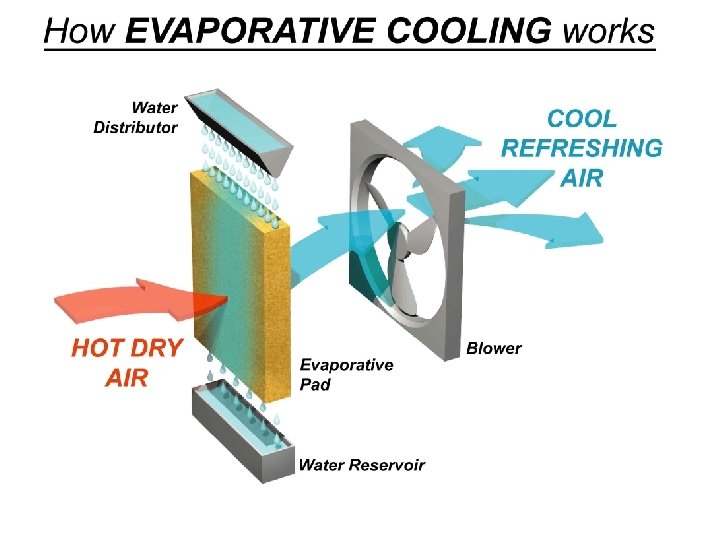
Evaporation • Evaporation is a cooling process.

Why doesn’t evaporating water freeze?

Evaporation is a cooling process

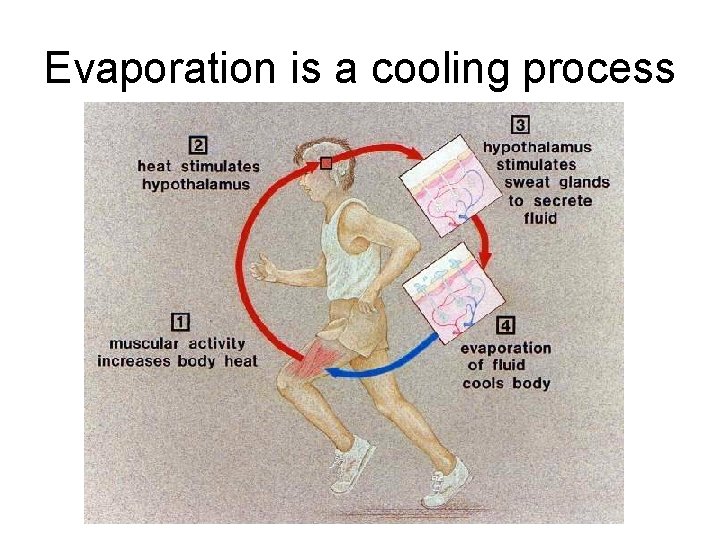
Evaporation is a cooling process


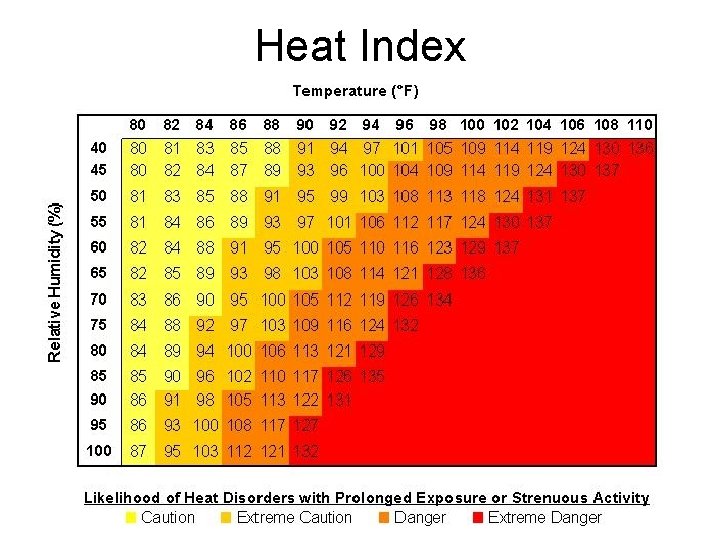
Heat Index

Evaporation is a cooling process


Saharan Desert

Saharan Oasis

Sahara was once fertile grasslands

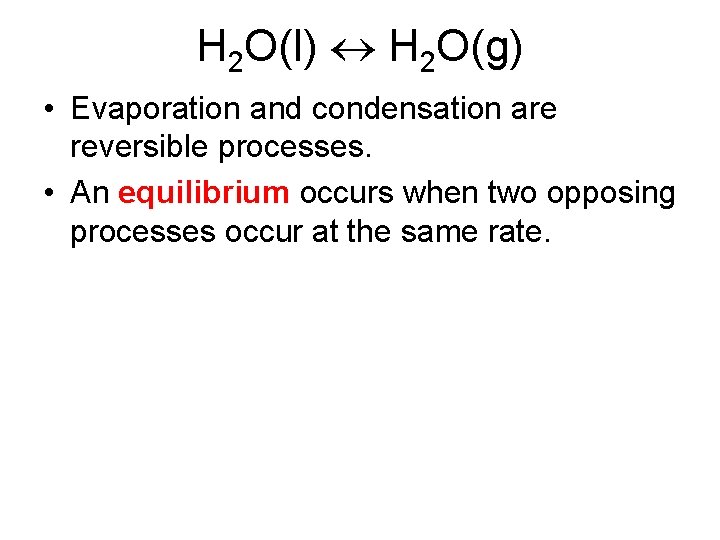
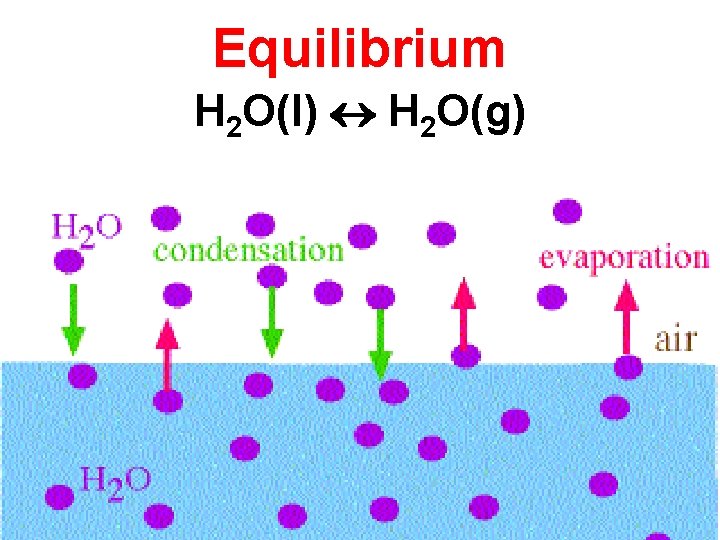
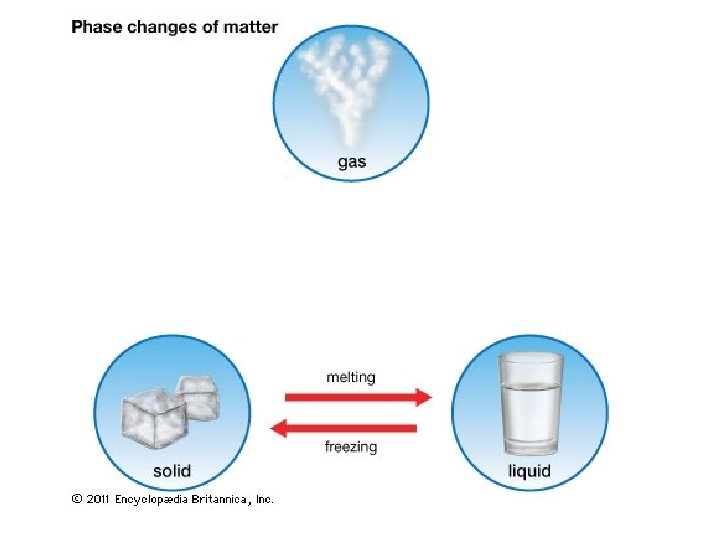
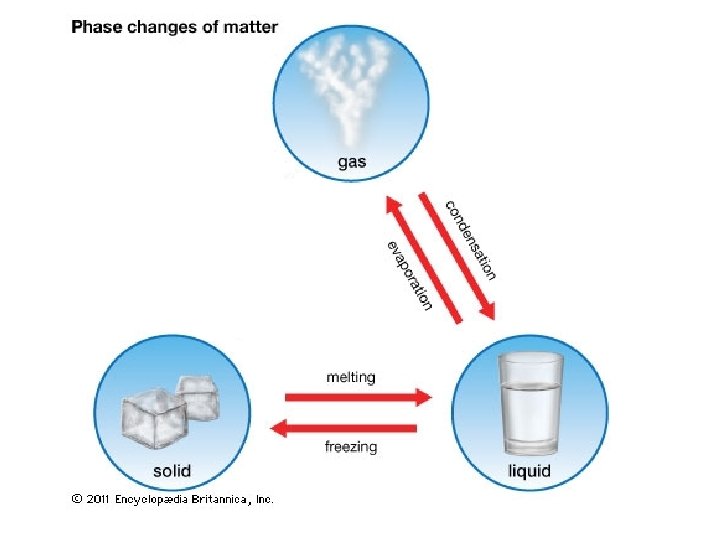
H 2 O(l) H 2 O(g) • Evaporation and condensation are reversible processes. • An equilibrium occurs when two opposing processes occur at the same rate.

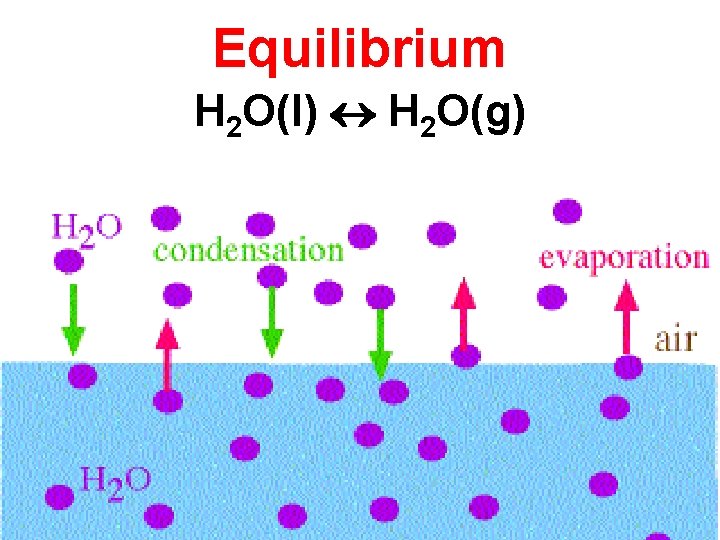
Equilibrium H 2 O(l) H 2 O(g)

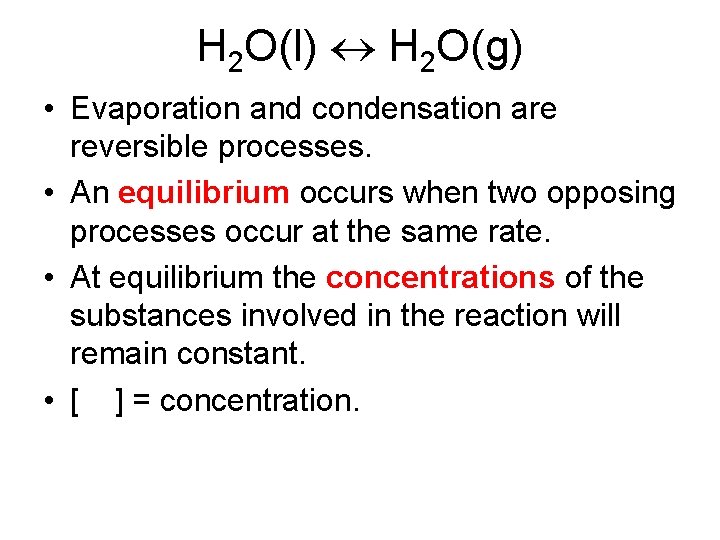
H 2 O(l) H 2 O(g) • Evaporation and condensation are reversible processes. • An equilibrium occurs when two opposing processes occur at the same rate. • At equilibrium the concentrations of the substances involved in the reaction will remain constant. • [ ] = concentration.

Equilibrium H 2 O(l) H 2 O(g)

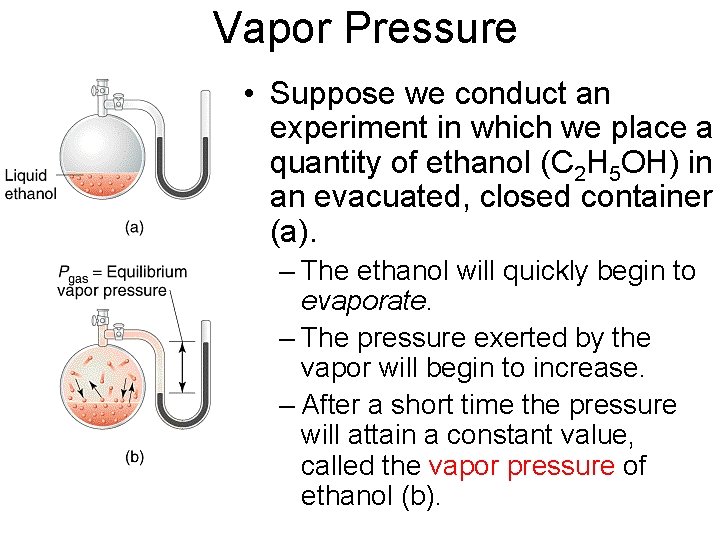
Vapor Pressure • Suppose we conduct an experiment in which we place a quantity of ethanol (C 2 H 5 OH) in an evacuated, closed container (a). – The ethanol will quickly begin to evaporate. – The pressure exerted by the vapor will begin to increase. – After a short time the pressure will attain a constant value, called the vapor pressure of ethanol (b).

Vapor Pressure • At any given temperature, for a particular substance, there is a pressure at which the gas of that substance is in equilibrium with its liquid or solid form. This is the vapor pressure of that substance at that temperature. • The equilibrium vapor pressure is an indication of a liquid's evaporation rate. • A substance with a high vapor pressure at normal temperatures has a high rate of evaporation and is often referred to as volatile.

Attractive Forces Two types of attractive forces are: 1. Intramolecular forces 2. Intermolecular forces

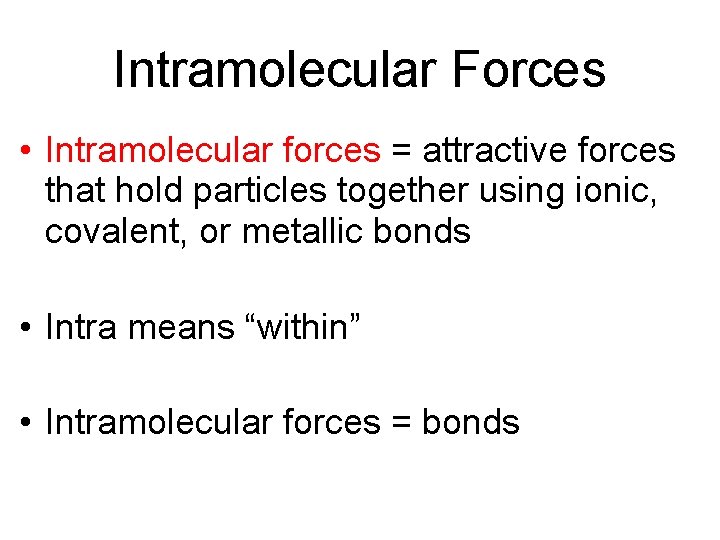
Intramolecular Forces • Intramolecular forces = attractive forces that hold particles together using ionic, covalent, or metallic bonds • Intra means “within” • Intramolecular forces = bonds

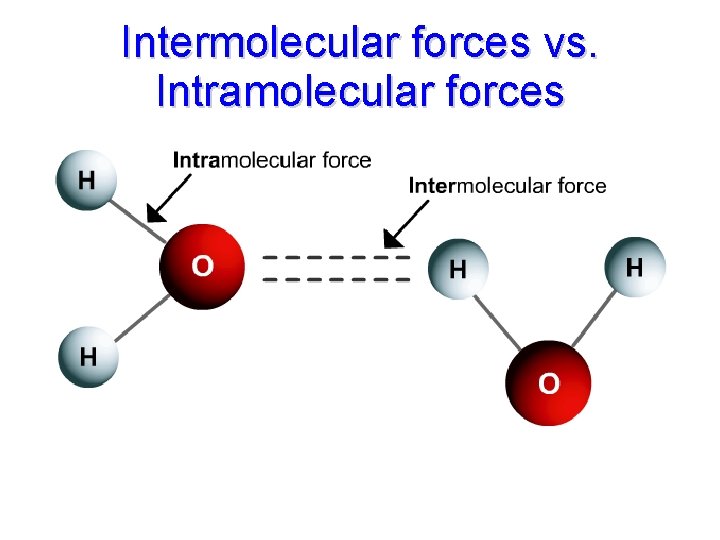
Intermolecular Forces (Van der Waals forces) • Inter means “between” • Intermolecular forces are forces between molecules • Larger polar molecules have greater Van der Waals forces.

Intermolecular forces vs. Intramolecular forces

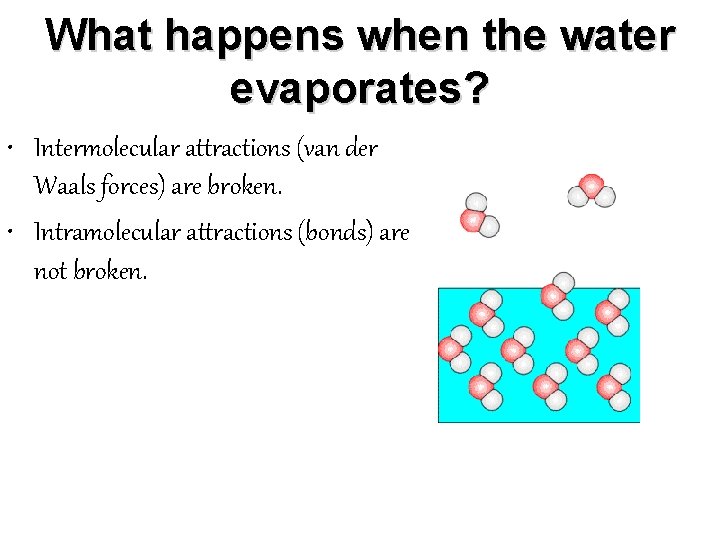
What happens when the water evaporates? • Intermolecular attractions (van der Waals forces) are broken. • Intramolecular attractions (bonds) are not broken.

alcohol vs. water How do the vapor pressures, rates of evaporation, and van der Waals forces compare? How would the boiling point of alcohol compare to water?

Why is it called rubbing alcohol?

Substances with weaker Van der Waals Forces 1. 2. 3. 4. Are easier to evaporate Have higher vapor pressure Are more volatile Have lower boiling points

Equilibrium • A state of equilibrium is the most stable state for a reversible system.

Le. Chatlier’s Principle • If a stress is placed on a system in equilibrium the system will tend to readjust so that the stress is reduced. • 3 Stresses are changing the: – Concentration – Temperature – Pressure • Le. Chatlier’s Principle = “Do the opposite”

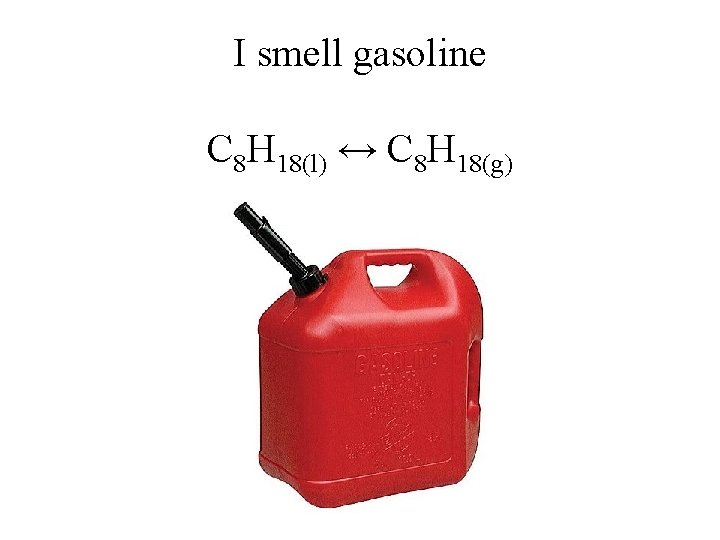
I smell gasoline C 8 H 18(l) ↔ C 8 H 18(g)

2 NO 2(g) ↔N 2 O 4(g) brown colorless How does applying Le. Chatlier’s Principle explain that this reaction is exothermic? Rule: An increase in temperature will always shift a reaction in the endothermic direction. What other rule could we use? Note: Apply the rule, it accounts for doing the opposite. So don’t do the opposite of the rule.

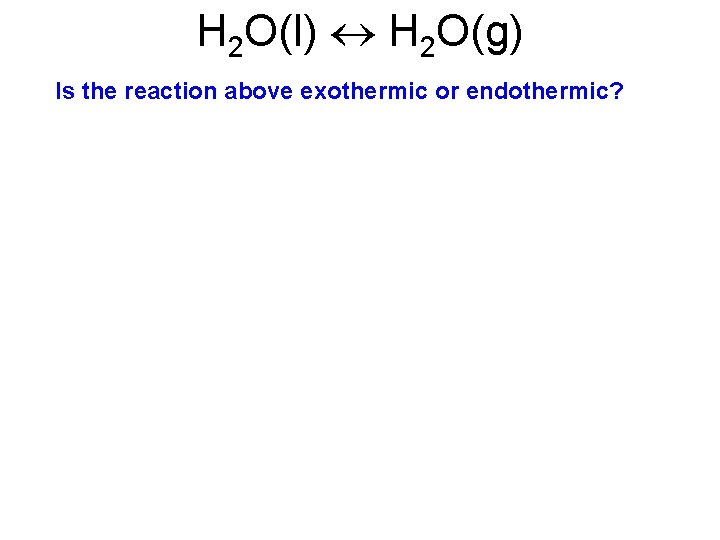
H 2 O(l) H 2 O(g) Is the reaction above exothermic or endothermic?

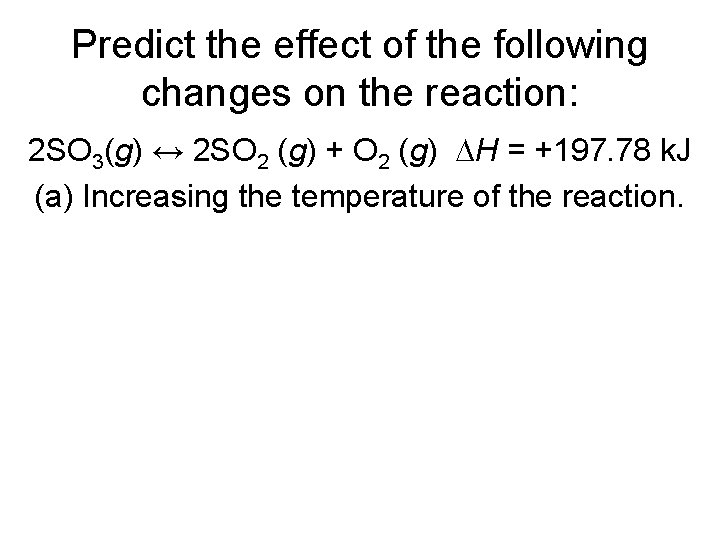
Predict the effect of the following changes on the reaction: 2 SO 3(g) ↔ 2 SO 2 (g) + O 2 (g) ∆H = +197. 78 k. J (a) Increasing the temperature of the reaction.

Predict the effect of the following changes on the reaction: 2 SO 3(g) ↔ 2 SO 2 (g) + O 2 (g) ∆H = +197. 78 k. J (b) Increasing the pressure on the reaction.

Predict the effect of the following changes on the reaction: 2 SO 3(g) ↔ 2 SO 2 (g) + O 2 (g) ∆H = +197. 78 k. J (c) Adding more O 2.

Predict the effect of the following changes on the reaction: 2 SO 3(g) ↔ 2 SO 2 (g) + O 2 (g) ∆H = +197. 78 k. J (d) Removing O 2.

Homework Worksheet 1 Chapter 17.





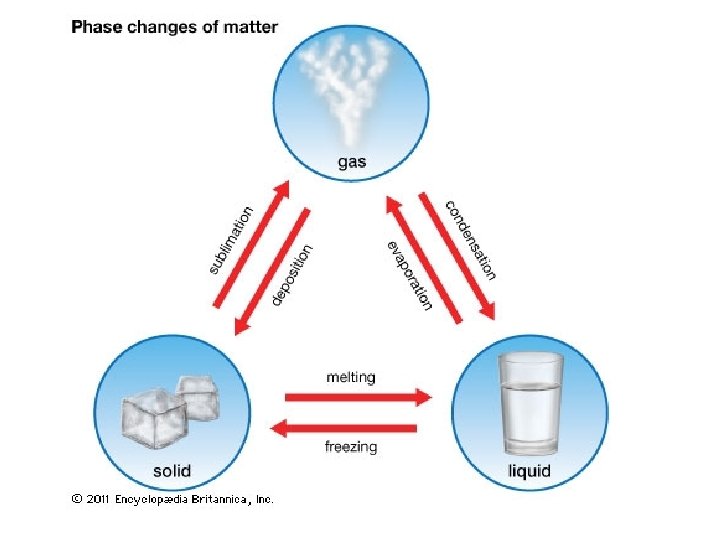
Solid-Vapor Equilibrium • Sublimation is a process in which molecules go directly from the solid into the vapor phase (deposition is the reverse process).

Sublimation Iodine and dry ice are substances that commonly undergo sublimation. Which of these can reach equilibrium?

Deposition • The formation of frost.

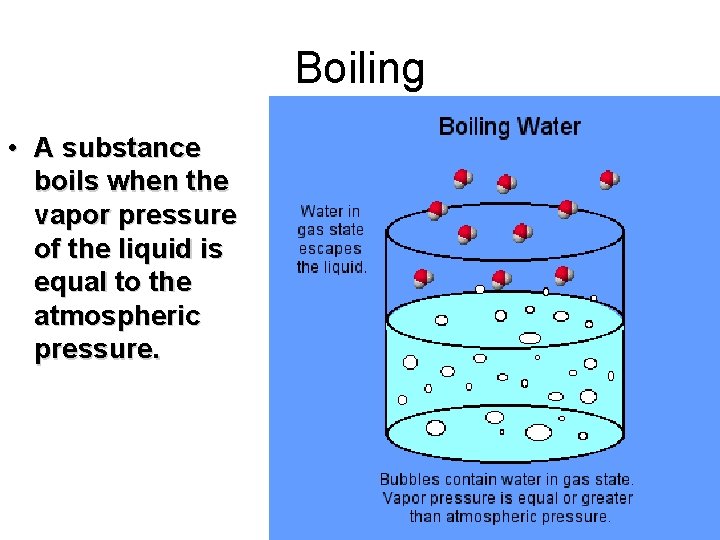
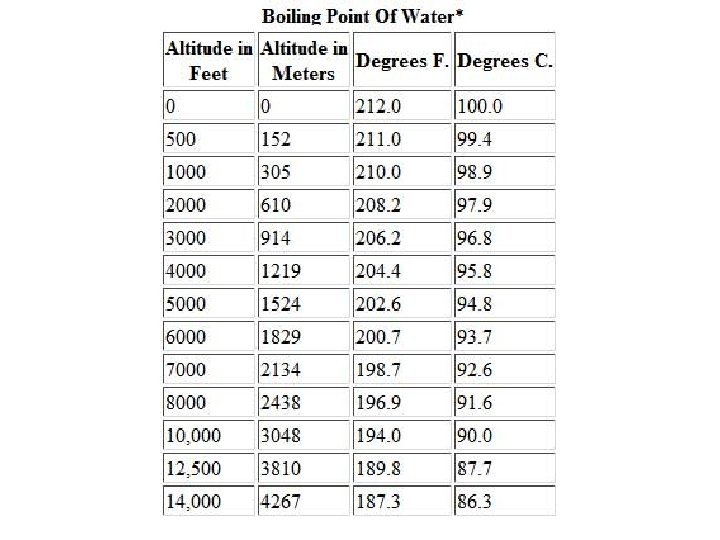
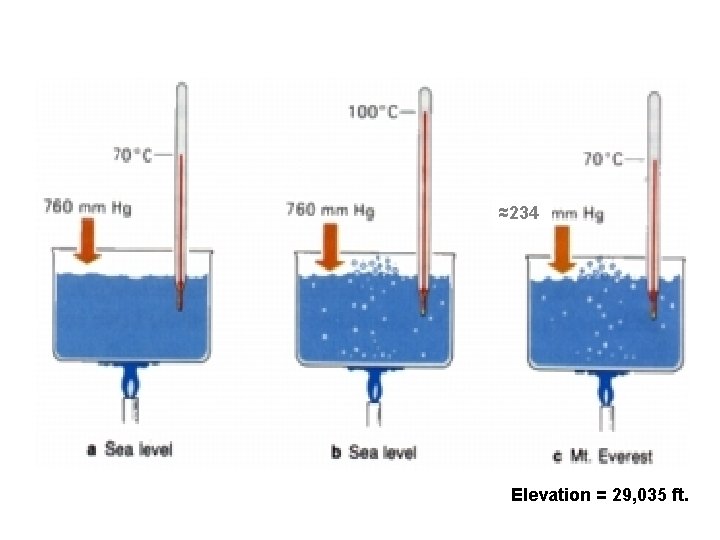
Boiling • A substance boils when the vapor pressure of the liquid is equal to the atmospheric pressure.

How could I boil this water? A A A

What is temperature of the water? A A A

Why does water boil at 100°C?

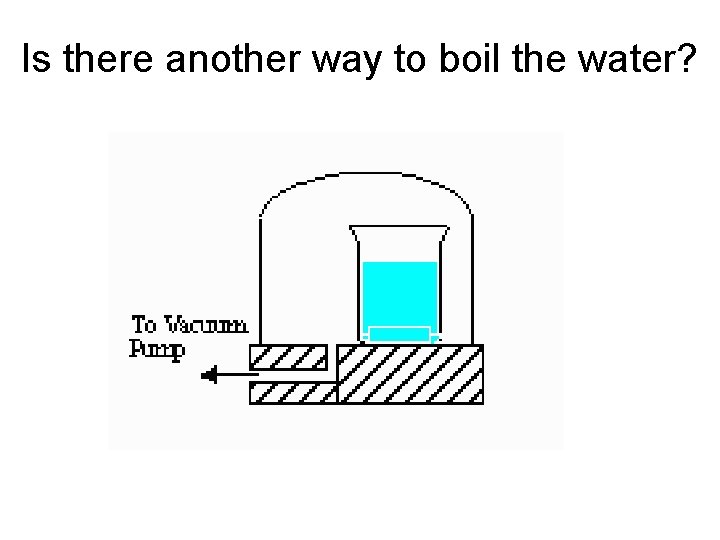
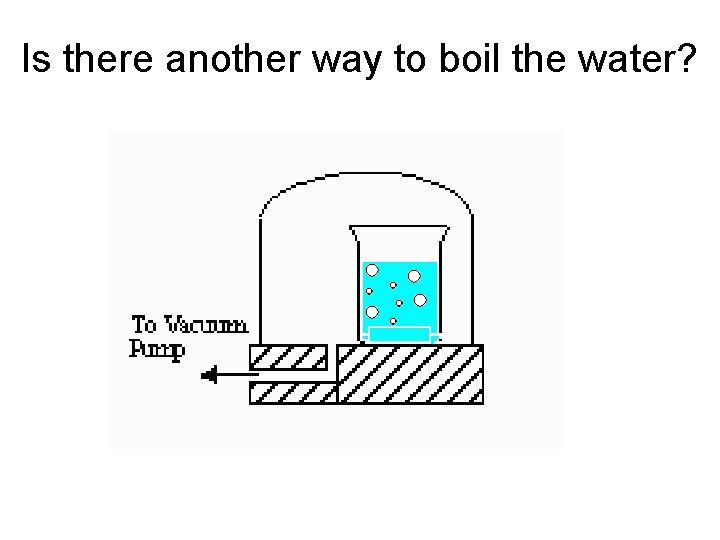
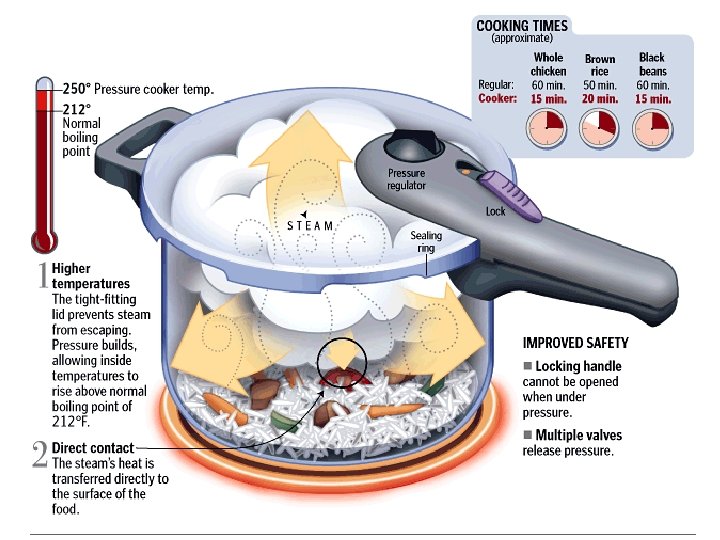
Is there another way to boil the water?

Is there another way to boil the water?

Is there another way to boil the water?

What is the pressure inside the bell jar if the water is boiling at 20°C?

We can make water boil at “any” temperature.


≈234 Elevation = 29, 035 ft.



Volcanic Vents

• LONDON — Scientists using a remote-controlled submarine have discovered the deepest known volcanic vent and say the superheated waters inside could contain undiscovered marine species and perhaps even clues to the origin of life on earth. • Experts aboard the RRS James Cook said they found the underwater volcanic vent more than three miles (five kilometers) beneath the surface of the Caribbean. • Volcanic vents areas where sea water seeps into small cracks that penetrate deep into the earth's crust — some reaching down more than a mile (two kilometers. ) Temperatures there can reach 750 degrees Fahrenheit (400 degrees Celsius), heating the water to the point where it can melt lead. • The blazing hot mineral-rich fluid is expelled into the icy cold of the deep ocean, creating a smoke-like effect and leaving behind towering chimneys of metal ore, some two stories tall. The spectacular pressure — 500 times stronger than the earth's atmosphere — keeps the water from boiling.

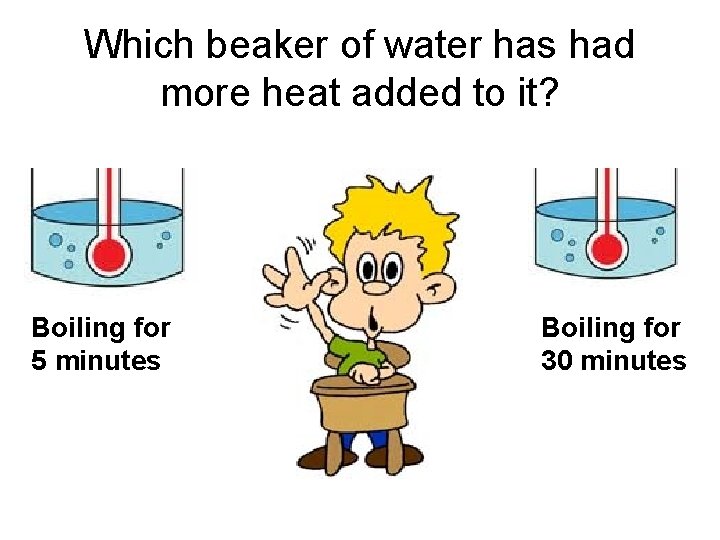
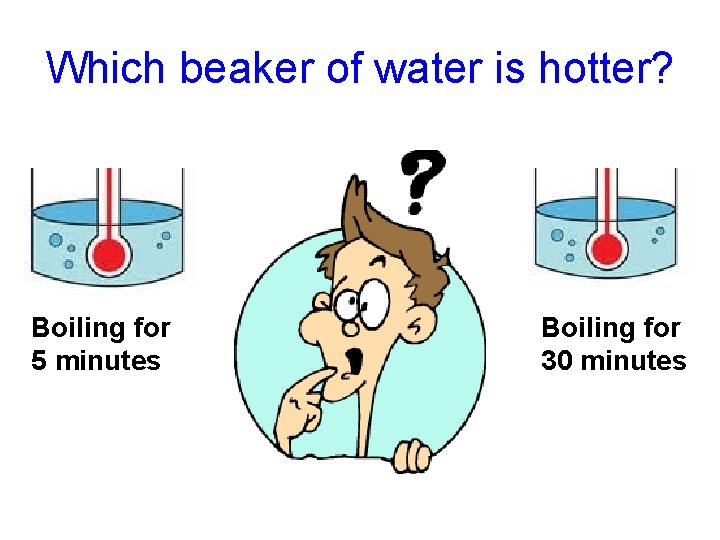
Which beaker of water has had more heat added to it? Boiling for 5 minutes Boiling for 30 minutes

Which beaker of water is hotter? Boiling for 5 minutes Boiling for 30 minutes

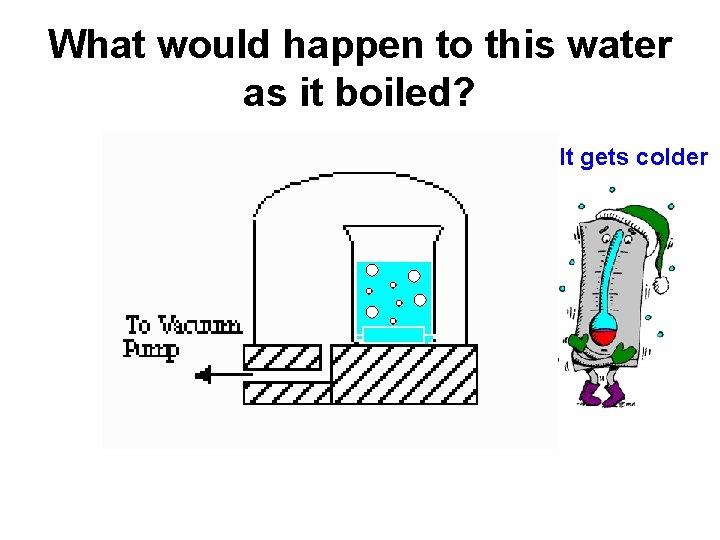
What would happen to this water as it boiled? It gets colder

How can we continue to boil the water as it cools?

What happens when the vapor pressure of water reaches 4. 6 mm Hg.



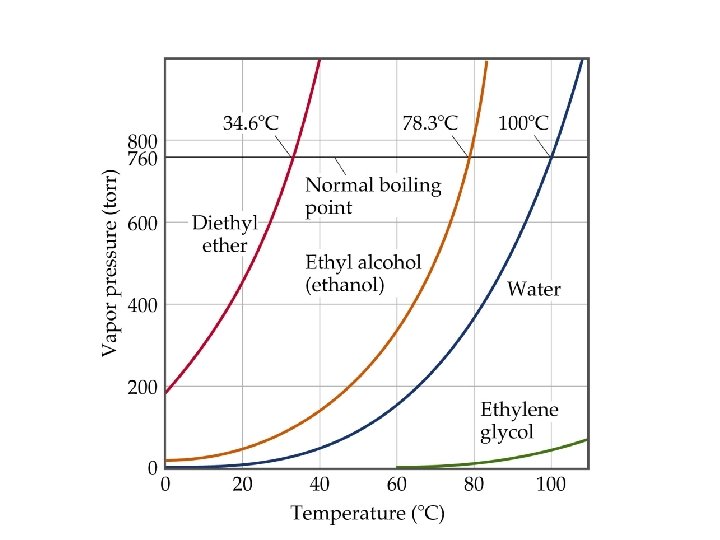
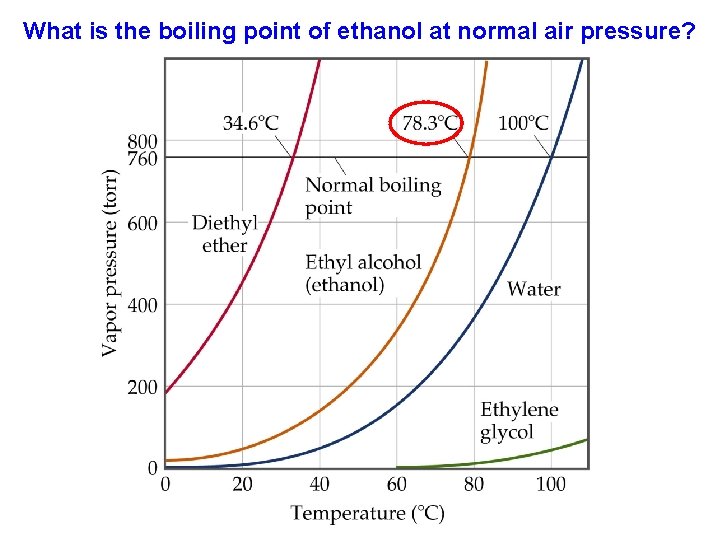
What is the boiling point of ethanol at normal air pressure?

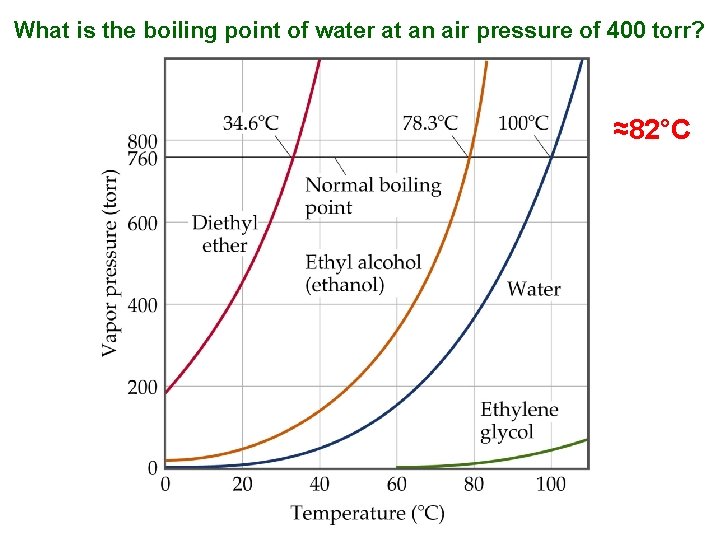
What is the boiling point of water at an air pressure of 400 torr? ≈82°C

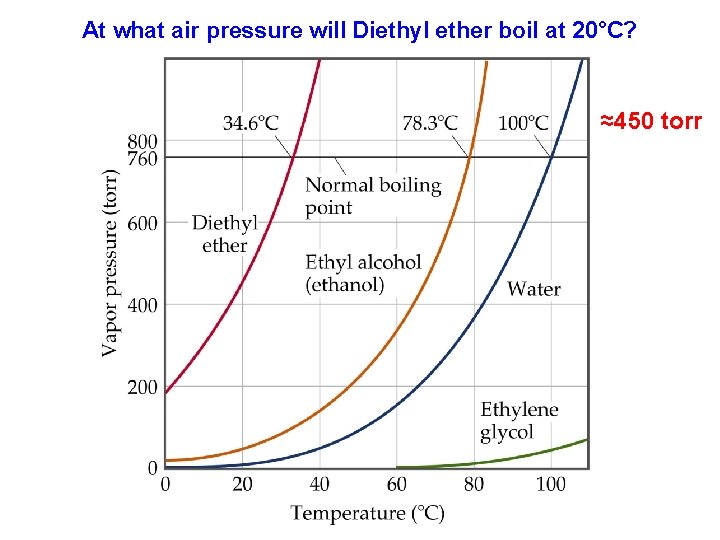
At what air pressure will Diethyl ether boil at 20°C? ≈450 torr

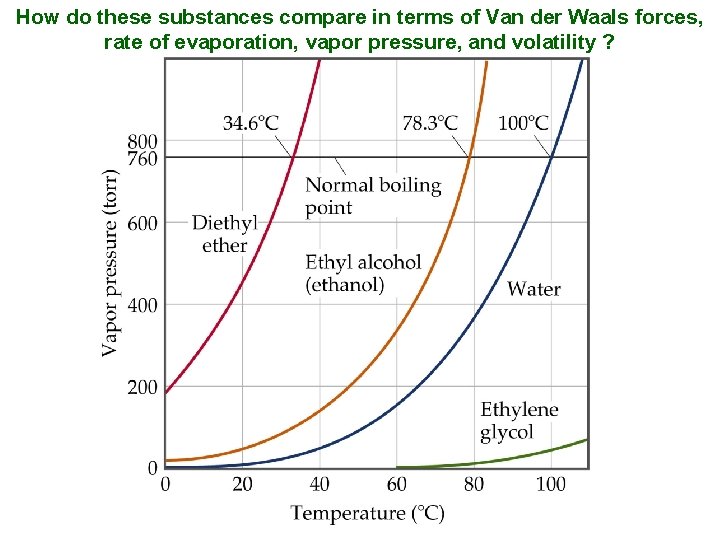
How do these substances compare in terms of Van der Waals forces, rate of evaporation, vapor pressure, and volatility ?

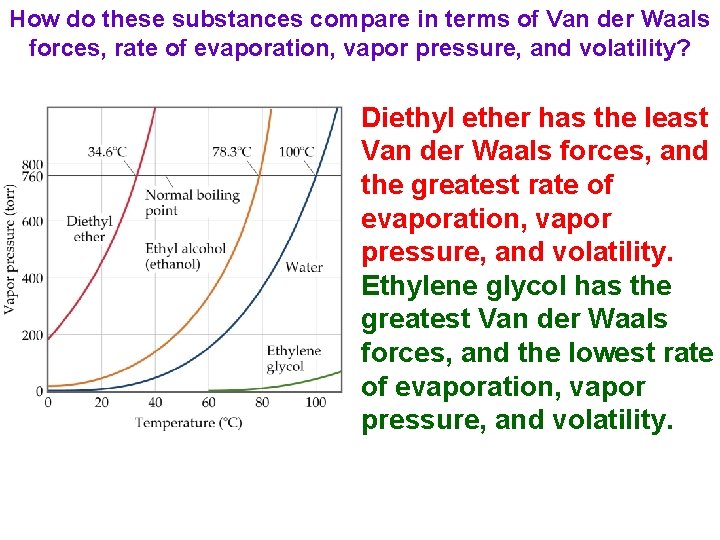
How do these substances compare in terms of Van der Waals forces, rate of evaporation, vapor pressure, and volatility? Diethyl ether has the least Van der Waals forces, and the greatest rate of evaporation, vapor pressure, and volatility. Ethylene glycol has the greatest Van der Waals forces, and the lowest rate of evaporation, vapor pressure, and volatility.

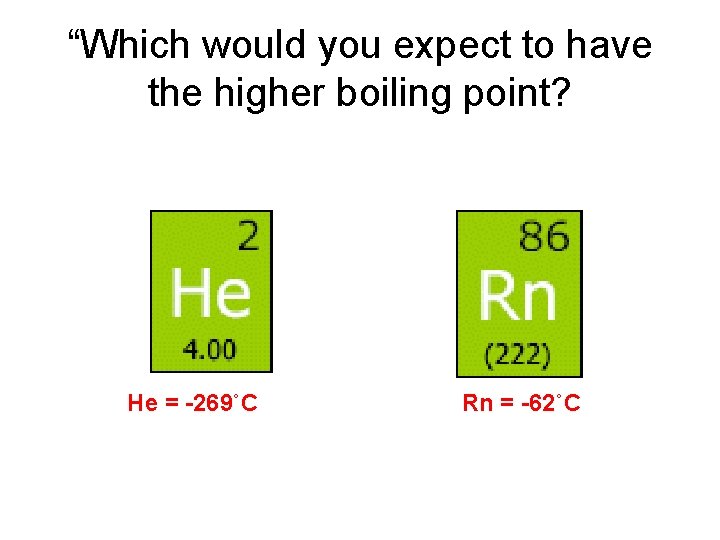
“Which would you expect to have the higher boiling point?

“Which would you expect to have the higher boiling point? He = -269˚C Rn = -62˚C

Demonstrations • Boiling water with by applying hands. • Freezing water by boiling.

Homework Summarize the “Equilibrium” Lab (due tomorrow). Worksheet 1 Chapter 17 (due tomorrow). Worksheet 2 Chapter 17 (due in two days). Study Guide Chapters 15 – 17 (due in two days).