Thermochemistry A Phase Changes A Phase Changes Evaporation



















![The specific heat (s) [most books use lower case c] of a substance is The specific heat (s) [most books use lower case c] of a substance is](https://slidetodoc.com/presentation_image_h2/0994f3e3db0689c97e45d3e35127703d/image-54.jpg)
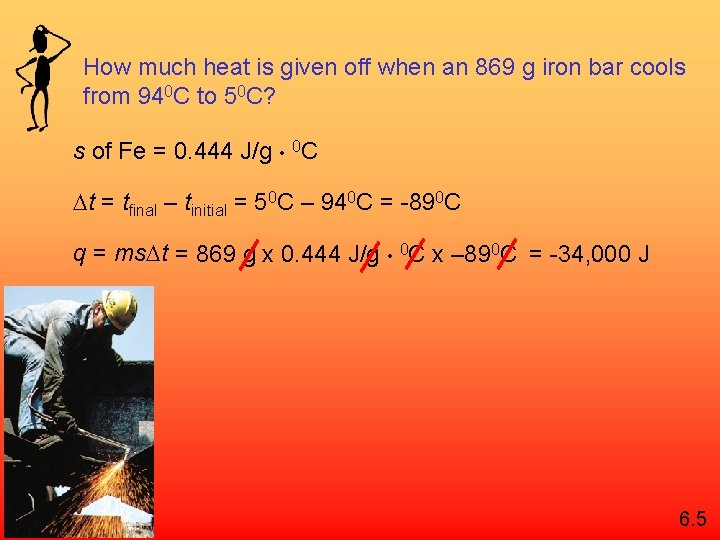
- Slides: 58

Thermochemistry

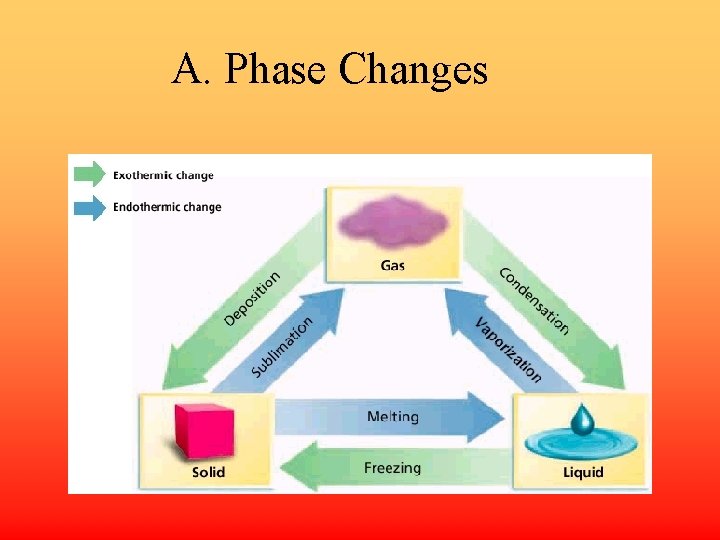
A. Phase Changes

A. Phase Changes Evaporation molecules at the surface gain enough energy to overcome IMF Volatility measure of evaporation rate depends on temp & IMF

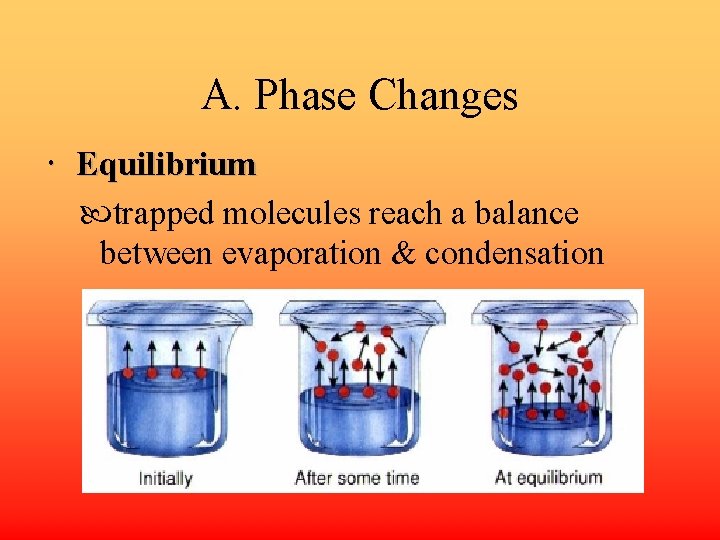
A. Phase Changes Equilibrium trapped molecules reach a balance between evaporation & condensation

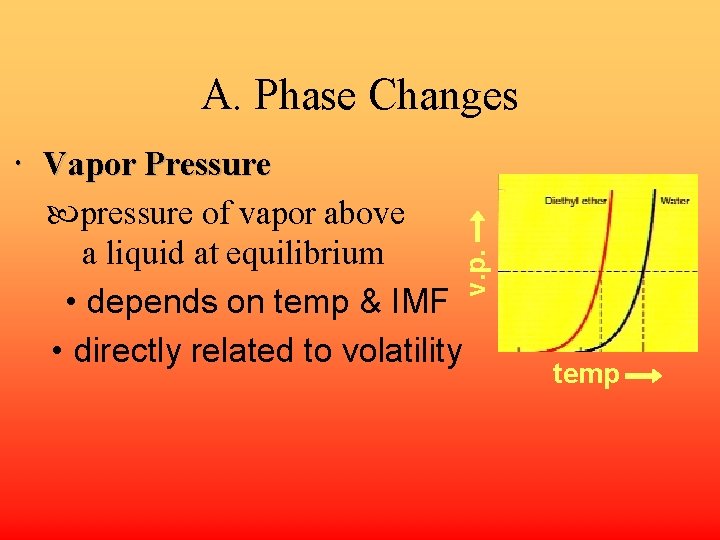
A. Phase Changes v. p. Vapor Pressure pressure of vapor above a liquid at equilibrium • depends on temp & IMF • directly related to volatility temp

A. Phase Changes • Boiling Point – temp at which v. p. of liquid equals external pressure • depends on Patm & IMF • Normal B. P. - b. p. at 1 atm

A. Phase Changes • Melting Point – equal to freezing point u Which has a higher m. p. ? • polar or nonpolar? polar • covalent or ionic? ionic

A. Phase Changes • Sublimation – solid gas – v. p. of solid equals external pressure u EX: dry ice, mothballs, solid air fresheners

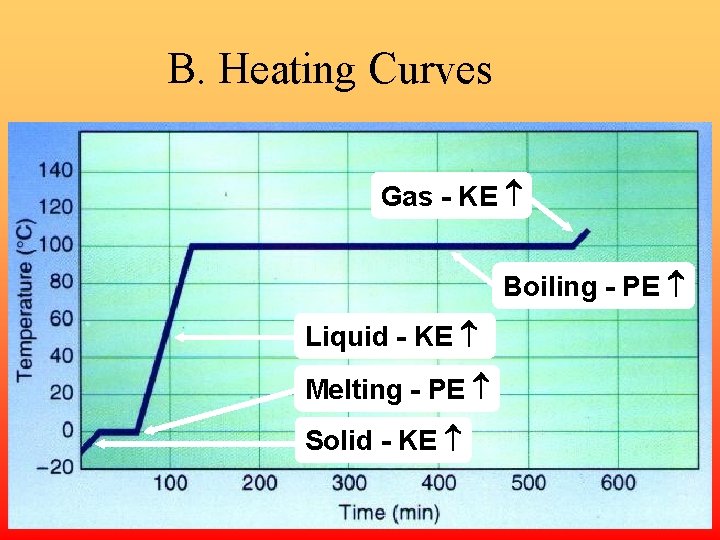
B. Heating Curves Gas - KE Boiling - PE Liquid - KE Melting - PE Solid - KE

B. Heating Curves • Temperature Change – change in KE (molecular motion) – depends on heat capacity u Heat Capacity • energy required to raise the temp of 1 gram of a substance by 1°C

B. Heating Curves • Phase Change – change in PE (molecular arrangement) – temp remains constant u Heat of Fusion ( Hfus) • energy required to melt 1 gram of a substance at its m. p.

B. Heating Curves • Heat of Vaporization ( Hvap) – energy required to boil 1 gram of a substance at its b. p. – usually larger than Hfus…why? u EX: sweating, steam burns, the drinking bird

C. Phase Diagrams • Show the phases of a substance at different temps and pressures.

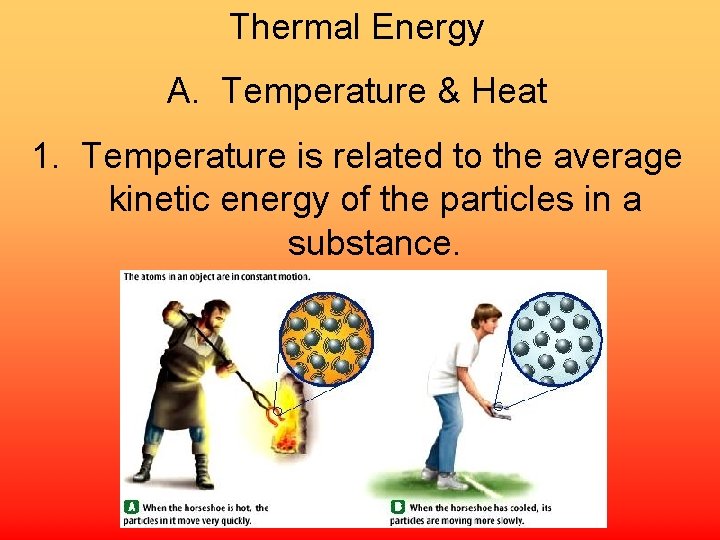
Thermal Energy A. Temperature & Heat 1. Temperature is related to the average kinetic energy of the particles in a substance.

2. SI unit for temp. is the Kelvin a. K = C + 273 (10 C = 283 K) b. C = K – 273 (10 K = -263 C) 3. Thermal Energy – the total of all the kinetic and potential energy of all the particles in a substance.

4. Thermal energy relationships a. As temperature increases, so does thermal energy (because the kinetic energy of the particles increased). b. Even if the temperature doesn’t change, thermal energy in a more massive substance is higher (because it is a total measure of energy).

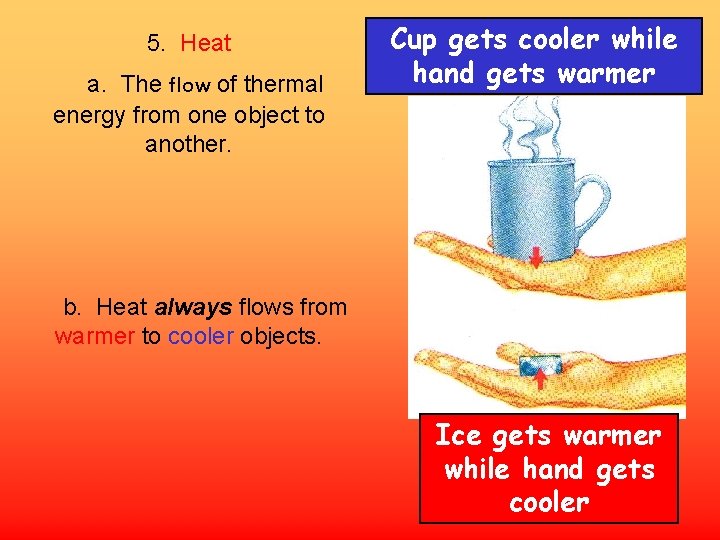
5. Heat a. The flow of thermal energy from one object to another. Cup gets cooler while hand gets warmer b. Heat always flows from warmer to cooler objects. Ice gets warmer while hand gets cooler

6. Specific Heat a. Some things heat up or cool down faster than others. Land heats up and cools down faster than water

b. Specific heat is the amount of heat required to raise the temperature of 1 kg of a material by one degree (C or K). 1) C water = 4184 J / kg C 2) C sand = 664 J / kg C This is why land heats up quickly during the day and cools quickly at night and why water takes longer.

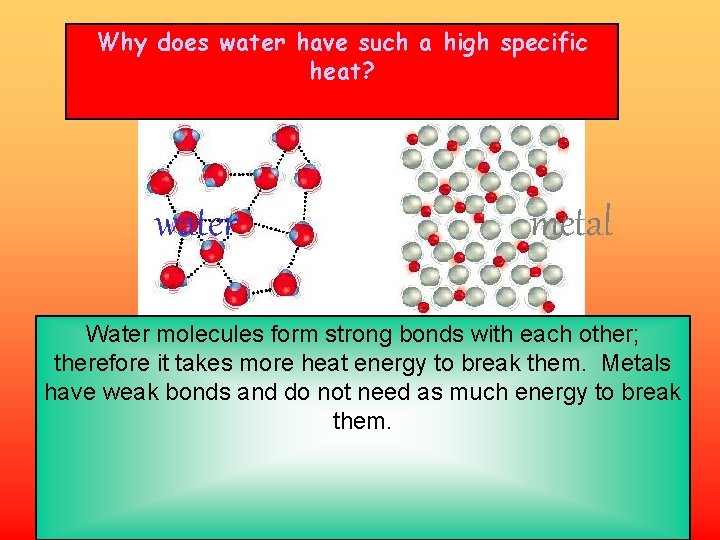
Why does water have such a high specific heat? water metal Water molecules form strong bonds with each other; therefore it takes more heat energy to break them. Metals have weak bonds and do not need as much energy to break them.

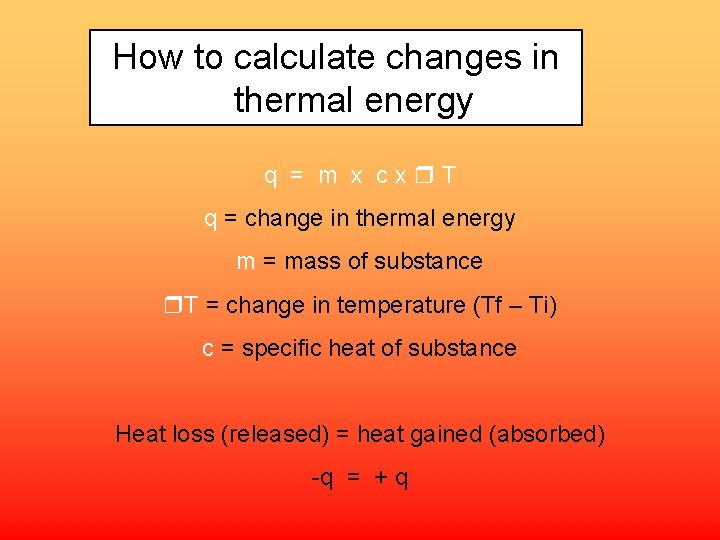
How to calculate changes in thermal energy q = m x cx T q = change in thermal energy m = mass of substance T = change in temperature (Tf – Ti) c = specific heat of substance Heat loss (released) = heat gained (absorbed) -q = + q

c. A calorimeter is used to help measure the specific heat of a substance. First, mass and temperature of water measured Knowing its are Q value, its mass, and its T, its Cp can be calculated Then sample put Thisheated gives the heatislost insidebyand flows into T is measured for water to theheat substance water help get its heat gain

HAPPY Monday! • PRE-AP Take out your notebooks!! Turn in any late work/check your boxes for returned quiz and returned redox homework. Heat Capacity Homework DUE WEDNESDAY REGULAR – Take out your Enthalpy Homework – we are going to go over some of the problems. – Enthalpy Hmk DUE TOMORROW. – Pre-TEST TOMORROW!! – TEST ON THURSDAY – REMINDERS – Project Description and Outline DUE FRIDAY! MAY 2 nd.

STUDY FOR PRE-TEST 1. Le. Chatelier’s Principle (Disney ride analogy!) 1. Add too much to L, move to right 2. Take away from L, move to left 2. Phase Changes 1. Heating Curve 2. Phase Change Diagram 3. Exothermic/Endothermic 4. Vocab for phase changes (sublimation etc)

3. Basic Temperature Vocabulary 1. Kinetic Energy and Potential Energy 2. Thermal Energy 3. Intermolecular forces

4. q and heat capacity problems 1. Q = amount of heat transferred (neg/pos) 2. C = specific heat 3. Units come from the C value q = mc T

5. Enthalpy Problems 1. H = (Products added) – (Reactants added) 2. Multiply by the coefficents 3. Elements in basic form are 0.

TWO Trends in Nature • Order Disorder • High energy Low energy

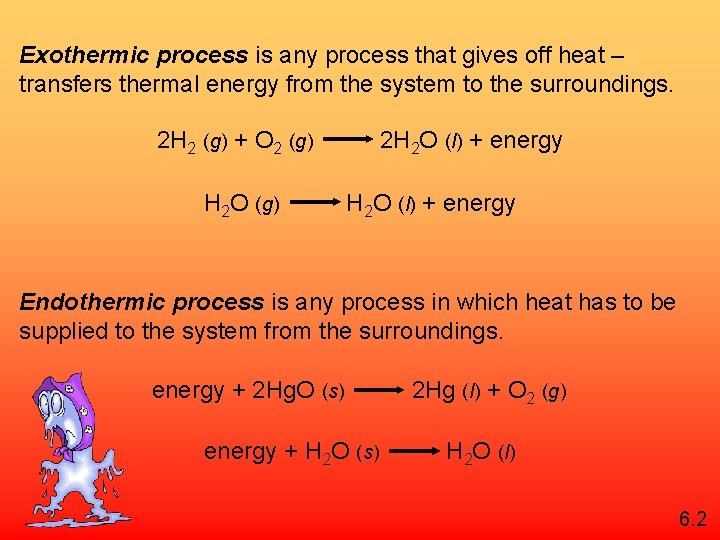
Exothermic process is any process that gives off heat – transfers thermal energy from the system to the surroundings. 2 H 2 (g) + O 2 (g) H 2 O (g) 2 H 2 O (l) + energy Endothermic process is any process in which heat has to be supplied to the system from the surroundings. energy + 2 Hg. O (s) energy + H 2 O (s) 2 Hg (l) + O 2 (g) H 2 O (l) 6. 2

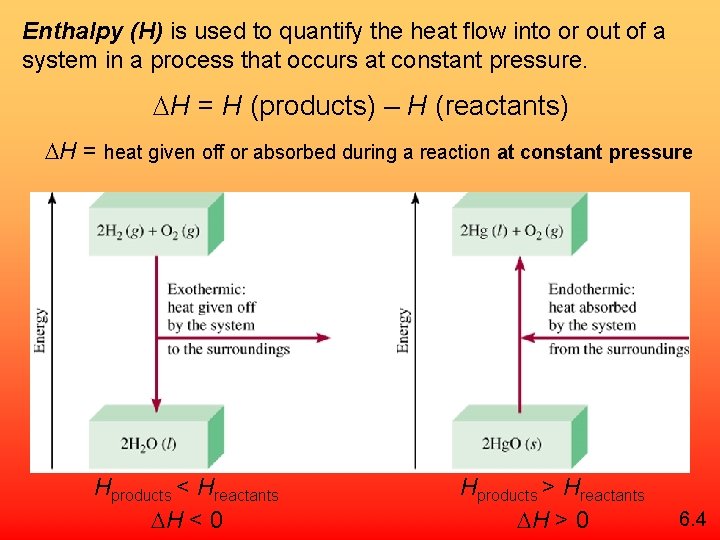
Enthalpy (H) is used to quantify the heat flow into or out of a system in a process that occurs at constant pressure. H = H (products) – H (reactants) H = heat given off or absorbed during a reaction at constant pressure Hproducts < Hreactants H < 0 Hproducts > Hreactants H > 0 6. 4

Thermochemical Equations Is H negative or positive? System absorbs heat Endothermic H > 0 and is positive 6. 01 k. J are absorbed for every 1 mole of ice that melts at 00 C and 1 atm. H 2 O (s) H 2 O (l) H = 6. 01 k. J 6. 4

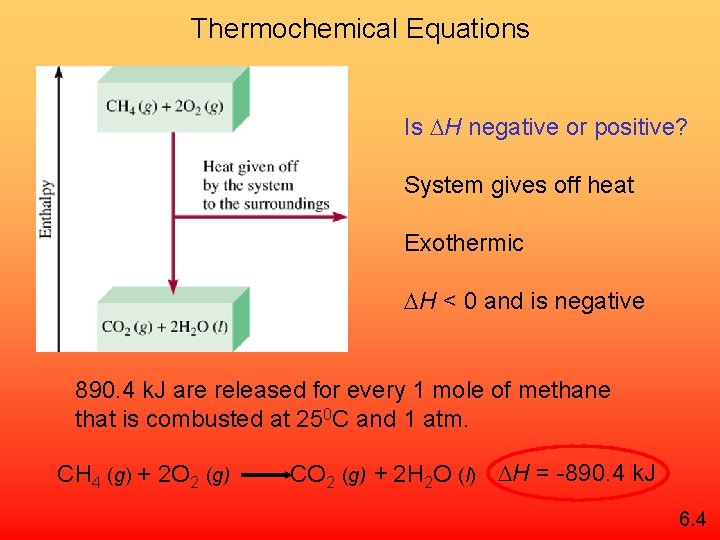
Thermochemical Equations Is H negative or positive? System gives off heat Exothermic H < 0 and is negative 890. 4 k. J are released for every 1 mole of methane that is combusted at 250 C and 1 atm. CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (l) H = -890. 4 k. J 6. 4

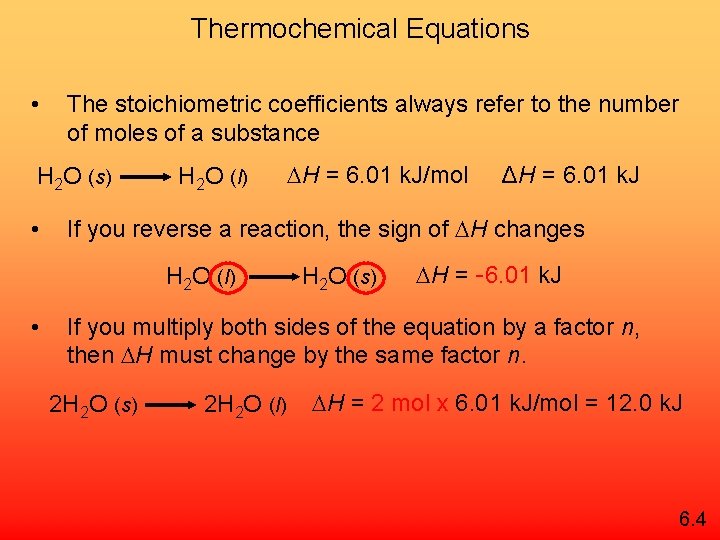
Thermochemical Equations • The stoichiometric coefficients always refer to the number of moles of a substance H 2 O (s) • H 2 O (l) ΔH = 6. 01 k. J If you reverse a reaction, the sign of H changes H 2 O (l) • H = 6. 01 k. J/mol H 2 O (s) H = -6. 01 k. J If you multiply both sides of the equation by a factor n, then H must change by the same factor n. 2 H 2 O (s) 2 H 2 O (l) H = 2 mol x 6. 01 k. J/mol = 12. 0 k. J 6. 4

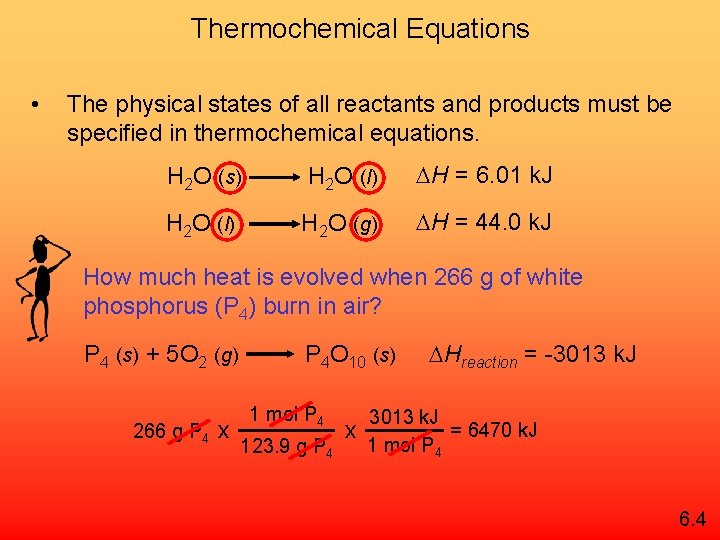
Thermochemical Equations • The physical states of all reactants and products must be specified in thermochemical equations. H 2 O (s) H 2 O (l) H = 6. 01 k. J H 2 O (l) H 2 O (g) H = 44. 0 k. J How much heat is evolved when 266 g of white phosphorus (P 4) burn in air? P 4 (s) + 5 O 2 (g) 266 g P 4 x P 4 O 10 (s) 1 mol P 4 123. 9 g P 4 x Hreaction = -3013 k. J = 6470 k. J 1 mol P 4 6. 4

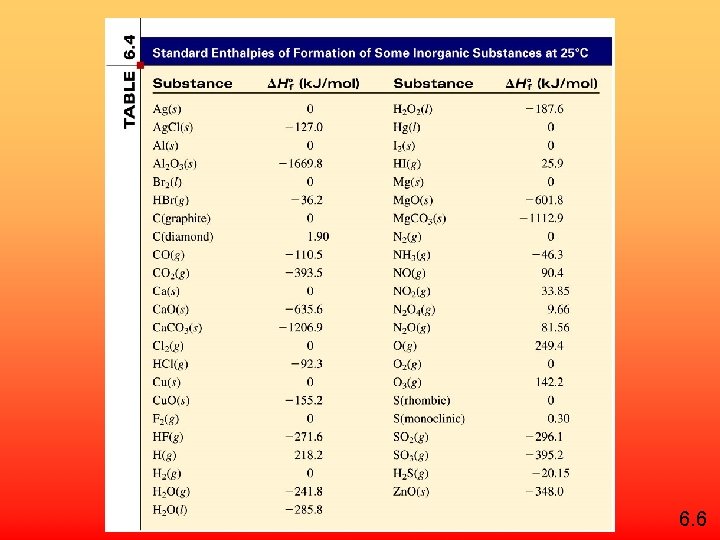
Standard enthalpy of formation ( Hf 0) is the heat change that results when one mole of a compound is formed from its elements at a pressure of 1 atm. The standard enthalpy of formation of any element in its most stable form is zero. H 0 f (O 2) = 0 H 0 f (C, graphite) = 0 H 0 f (O 3) = 142 k. J/mol H 0 f (C, diamond) = 1. 90 k. J/mol 6. 6

6. 6

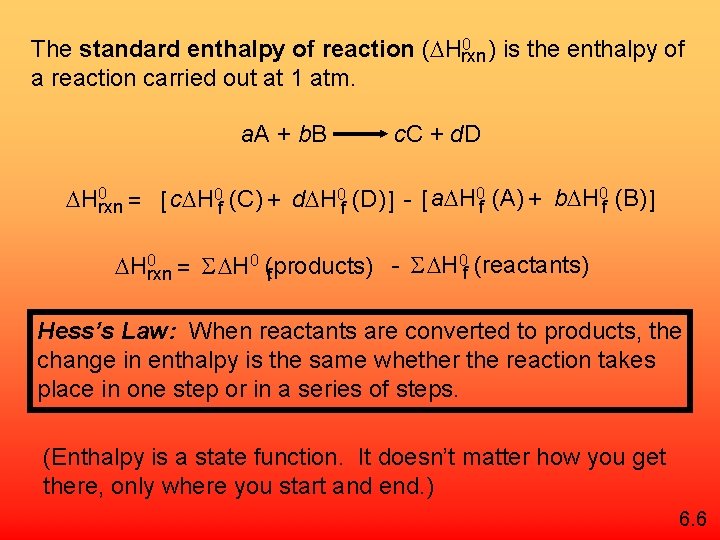
0 ) is the enthalpy of The standard enthalpy of reaction ( Hrxn a reaction carried out at 1 atm. a. A + b. B c. C + d. D H 0 rxn = [ c H 0 f (C) + d H 0 f (D) ] - [ a H 0 f (A) + b H 0 f (B) ] 0 (reactants) H S H 0 rxn = S H 0 (products) f f Hess’s Law: When reactants are converted to products, the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps. (Enthalpy is a state function. It doesn’t matter how you get there, only where you start and end. ) 6. 6

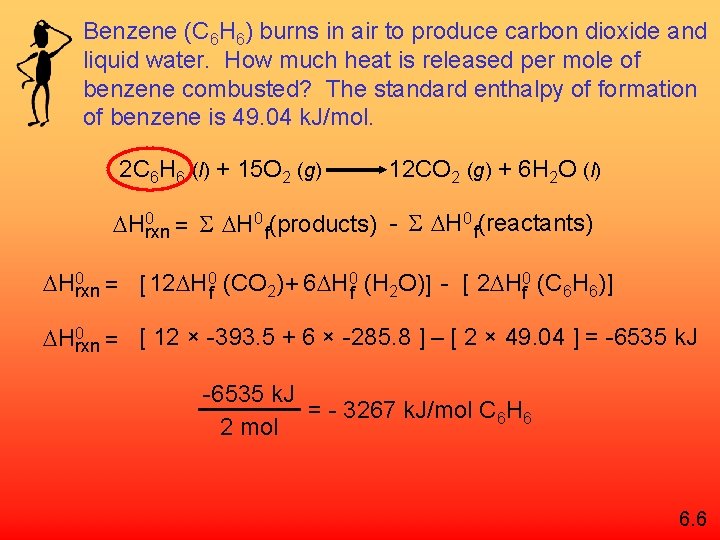
Benzene (C 6 H 6) burns in air to produce carbon dioxide and liquid water. How much heat is released per mole of benzene combusted? The standard enthalpy of formation of benzene is 49. 04 k. J/mol. 2 C 6 H 6 (l) + 15 O 2 (g) 12 CO 2 (g) + 6 H 2 O (l) H 0 rxn = S H 0 f(products) - S H 0 f(reactants) H 0 rxn = [ 12 H 0 f (CO 2) + 6 H 0 f (H 2 O)] - [ 2 H 0 f (C 6 H 6)] H 0 rxn = [ 12 × -393. 5 + 6 × -285. 8 ] – [ 2 × 49. 04 ] = -6535 k. J = - 3267 k. J/mol C 6 H 6 2 mol 6. 6

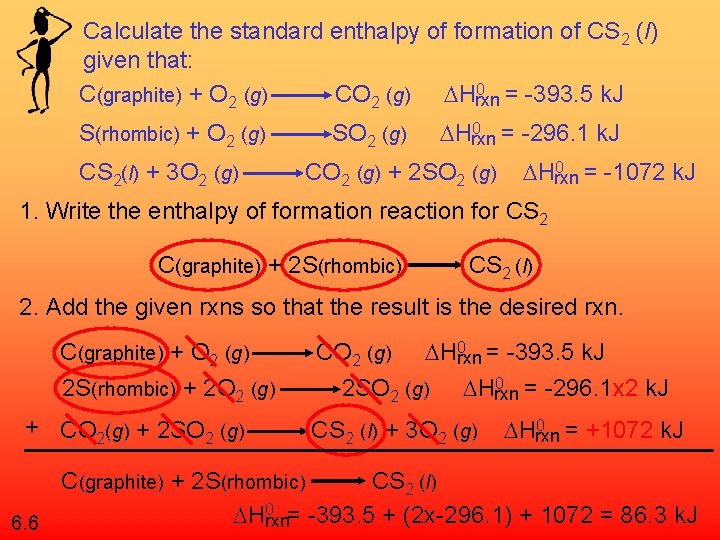
Calculate the standard enthalpy of formation of CS 2 (l) given that: 0 = -393. 5 k. J C(graphite) + O 2 (g) CO 2 (g) Hrxn S(rhombic) + O 2 (g) CS 2(l) + 3 O 2 (g) SO 2 (g) 0 = -296. 1 k. J Hrxn CO 2 (g) + 2 SO 2 (g) 0 = -1072 k. J Hrxn 1. Write the enthalpy of formation reaction for CS 2 C(graphite) + 2 S(rhombic) CS 2 (l) 2. Add the given rxns so that the result is the desired rxn. C(graphite) + O 2 (g) 2 S(rhombic) + 2 O 2 (g) + CO 2(g) + 2 SO 2 (g) 0 = -393. 5 k. J CO 2 (g) Hrxn 0 = -296. 1 x 2 k. J 2 SO 2 (g) Hrxn CS 2 (l) + 3 O 2 (g) 0 = +1072 k. J Hrxn C(graphite) + 2 S(rhombic) CS 2 (l) 0 = -393. 5 + (2 x-296. 1) + 1072 = 86. 3 k. J H rxn 6. 6

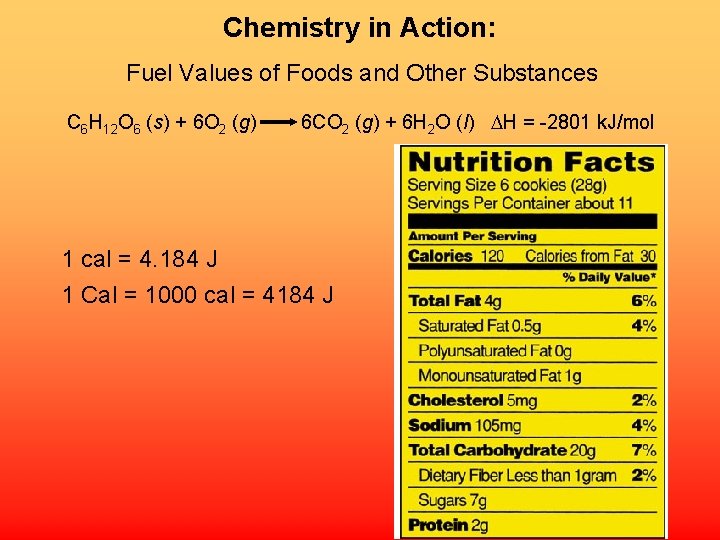
Chemistry in Action: Fuel Values of Foods and Other Substances C 6 H 12 O 6 (s) + 6 O 2 (g) 6 CO 2 (g) + 6 H 2 O (l) H = -2801 k. J/mol 1 cal = 4. 184 J 1 Cal = 1000 cal = 4184 J

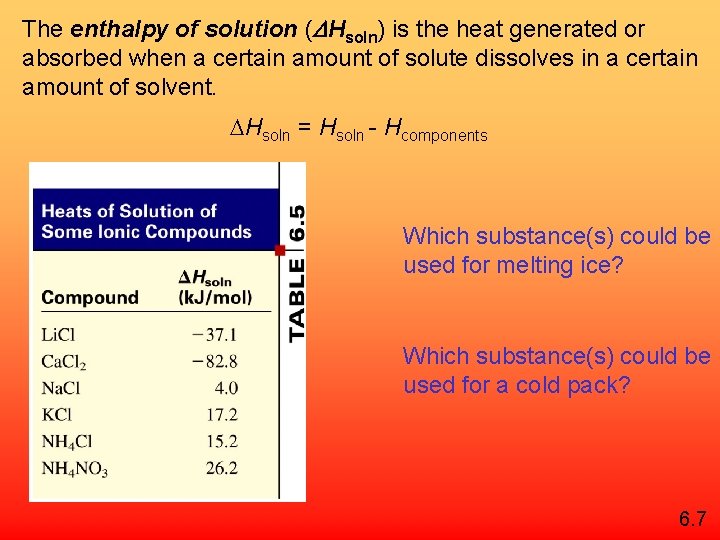
The enthalpy of solution (DHsoln) is the heat generated or absorbed when a certain amount of solute dissolves in a certain amount of solvent. Hsoln = Hsoln - Hcomponents Which substance(s) could be used for melting ice? Which substance(s) could be used for a cold pack? 6. 7

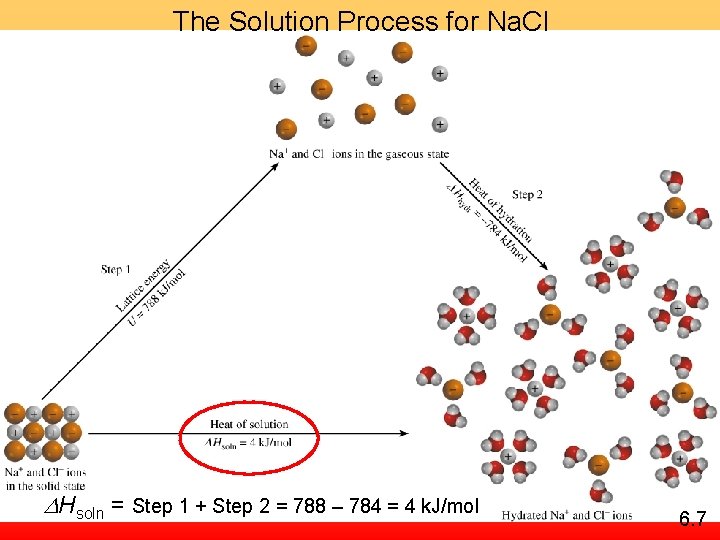
The Solution Process for Na. Cl DHsoln = Step 1 + Step 2 = 788 – 784 = 4 k. J/mol 6. 7

Energy Diagrams Exothermic Endothermic (a) Activation energy (Ea) for the forward reaction 50 k. J/mol 300 k. J/mol (b) Activation energy (Ea) for the reverse reaction 150 k. J/mol 100 k. J/mol (c) Delta H -100 k. J/mol +200 k. J/mol

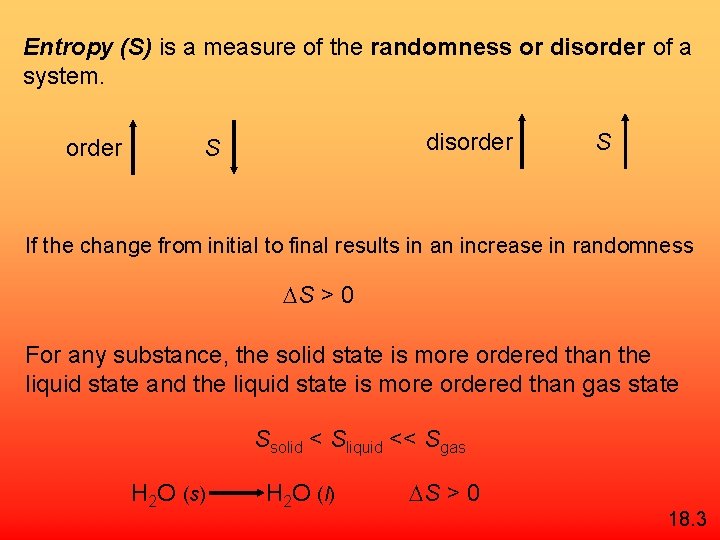
Entropy (S) is a measure of the randomness or disorder of a system. order disorder S S If the change from initial to final results in an increase in randomness S > 0 For any substance, the solid state is more ordered than the liquid state and the liquid state is more ordered than gas state Ssolid < Sliquid << Sgas H 2 O (s) H 2 O (l) S > 0 18. 3

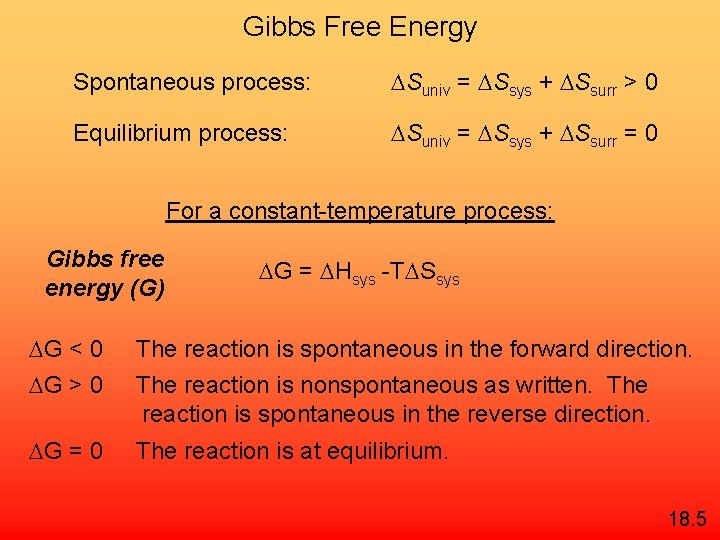
First Law of Thermodynamics Energy can be converted from one form to another but energy cannot be created or destroyed. Second Law of Thermodynamics The entropy of the universe increases in a spontaneous process and remains unchanged in an equilibrium process. Spontaneous process: Suniv = Ssys + Ssurr > 0 Equilibrium process: Suniv = Ssys + Ssurr = 0 18. 4

Entropy Changes in the System ( Ssys) The standard entropy of reaction ( S 0 rxn) is the entropy change for a reaction carried out at 1 atm and 250 C. a. A + b. B S 0 rxn = c. C + d. D [ c. S 0(C) + d. S 0(D) ] - [ a. S 0(A) + b. S 0(B) ] S 0 rxn = S S 0(products) - S S 0(reactants) What is the standard entropy change for the following reaction at 250 C? 2 CO (g) + O 2 (g) 2 CO 2 (g) S 0(CO) = 197. 9 J/K • mol S 0(O 2) = 205. 0 J/K • mol S 0(CO 2) = 213. 6 J/K • mol S 0 rxn = 2 x S 0(CO 2) – [2 x S 0(CO) + S 0 (O 2)] S 0 rxn = 427. 2 – [395. 8 + 205. 0] = -173. 6 J/K • mol 18. 4

Entropy Changes in the System ( Ssys) When gases are produced (or consumed) • If a reaction produces more gas molecules than it consumes, S 0 > 0. • If the total number of gas molecules diminishes, S 0 < 0. • If there is no net change in the total number of gas molecules, then S 0 may be positive or negative BUT S 0 will be a small number. What is the sign of the entropy change for the following reaction? 2 Zn (s) + O 2 (g) 2 Zn. O (s) The total number of gas molecules goes down, S is negative. 18. 4

Spontaneous Physical and Chemical Processes • A waterfall runs downhill • A lump of sugar dissolves in a cup of coffee • At 1 atm, water freezes below 0 0 C and ice melts above 0 0 C • Heat flows from a hotter object to a colder object • A gas expands in an evacuated bulb • Iron exposed to oxygen and water forms rust spontaneous nonspontaneous 18. 2

Gibbs Free Energy Spontaneous process: Suniv = Ssys + Ssurr > 0 Equilibrium process: Suniv = Ssys + Ssurr = 0 For a constant-temperature process: Gibbs free energy (G) G = Hsys -T Ssys G < 0 The reaction is spontaneous in the forward direction. G > 0 The reaction is nonspontaneous as written. The reaction is spontaneous in the reverse direction. G = 0 The reaction is at equilibrium. 18. 5

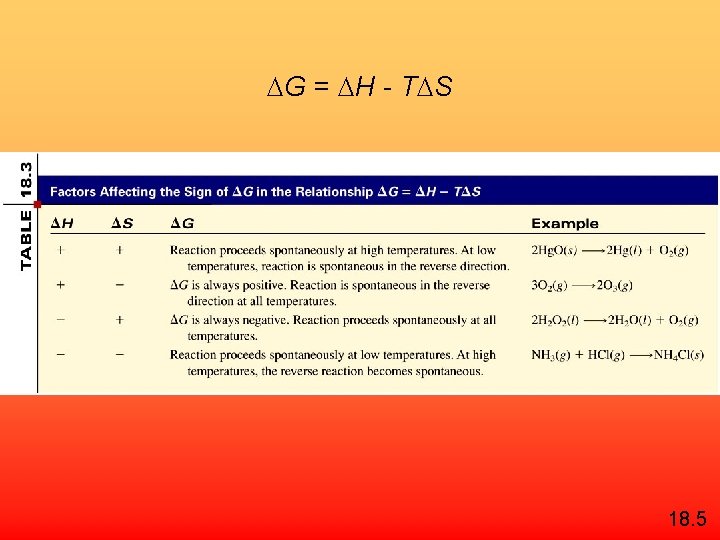
G = H - T S 18. 5

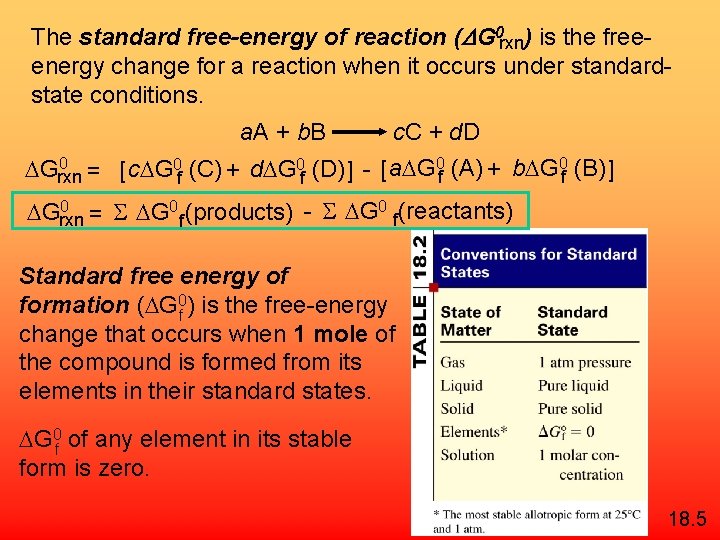
The standard free-energy of reaction (DG 0 rxn) is the freeenergy change for a reaction when it occurs under standardstate conditions. a. A + b. B c. C + d. D 0 Grxn = [ c G 0 f (C) + d G 0 f (D) ] - [ a G 0 f (A) + b G 0 f (B) ] 0 Grxn = S G 0 f (products) - S G 0 f(reactants) Standard free energy of formation ( G 0 f ) is the free-energy change that occurs when 1 mole of the compound is formed from its elements in their standard states. G 0 f of any element in its stable form is zero. 18. 5

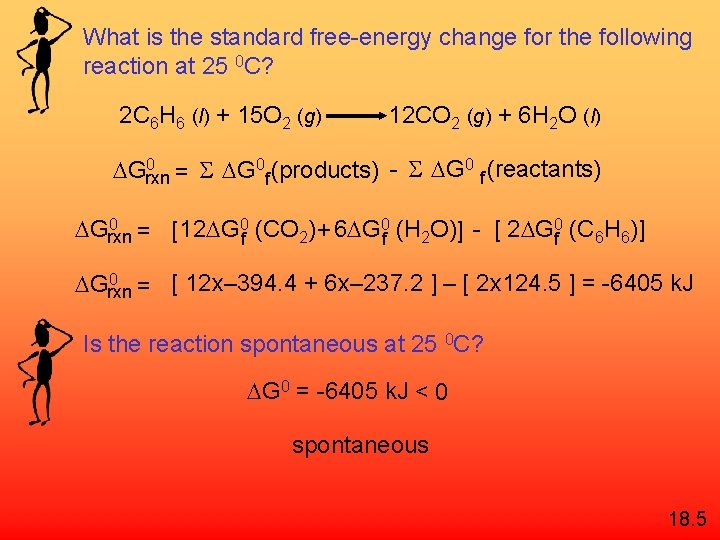
What is the standard free-energy change for the following reaction at 25 0 C? 2 C 6 H 6 (l) + 15 O 2 (g) 12 CO 2 (g) + 6 H 2 O (l) 0 Grxn = S G 0 f (products) - S G 0 f (reactants) 0 Grxn = [12 G 0 f (CO 2) + 6 G 0 f (H 2 O)] - [ 2 G 0 f (C 6 H 6)] 0 Grxn = [ 12 x– 394. 4 + 6 x– 237. 2 ] – [ 2 x 124. 5 ] = -6405 k. J Is the reaction spontaneous at 25 0 C? G 0 = -6405 k. J < 0 spontaneous 18. 5

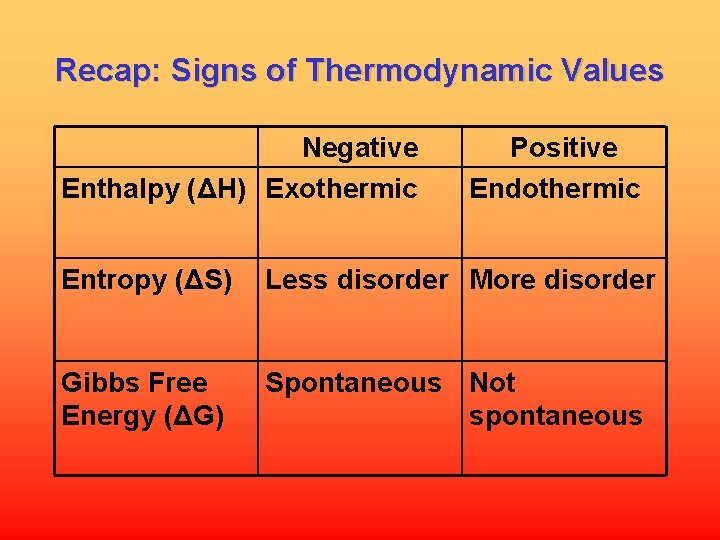
Recap: Signs of Thermodynamic Values Negative Enthalpy (ΔH) Exothermic Positive Endothermic Entropy (ΔS) Less disorder More disorder Gibbs Free Energy (ΔG) Spontaneous Not spontaneous
![The specific heat s most books use lower case c of a substance is The specific heat (s) [most books use lower case c] of a substance is](https://slidetodoc.com/presentation_image_h2/0994f3e3db0689c97e45d3e35127703d/image-54.jpg)
The specific heat (s) [most books use lower case c] of a substance is the amount of heat (q) required to raise the temperature of one gram of the substance by one degree Celsius. The heat capacity (C) of a substance is the amount of heat (q) required to raise the temperature of a given quantity (m) of the substance by one degree Celsius. C = ms Heat (q) absorbed or released: q = ms t q = C t t = tfinal - tinitial 6. 5
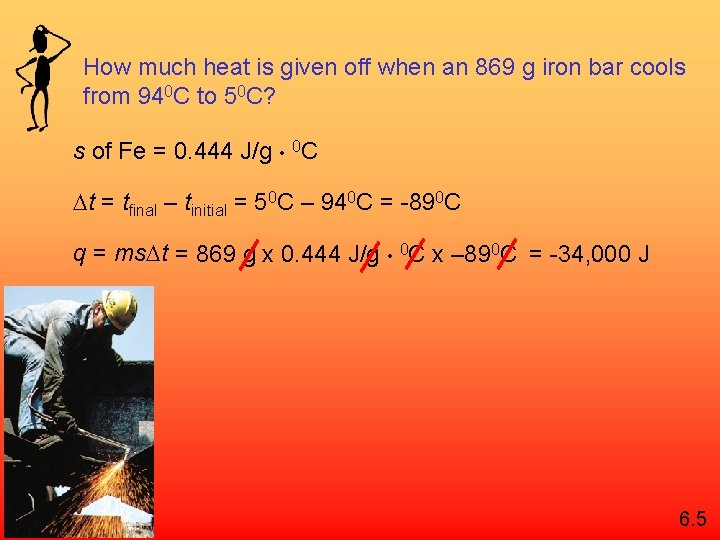
How much heat is given off when an 869 g iron bar cools from 940 C to 50 C? s of Fe = 0. 444 J/g • 0 C t = tfinal – tinitial = 50 C – 940 C = -890 C q = ms t = 869 g x 0. 444 J/g • 0 C x – 890 C = -34, 000 J 6. 5

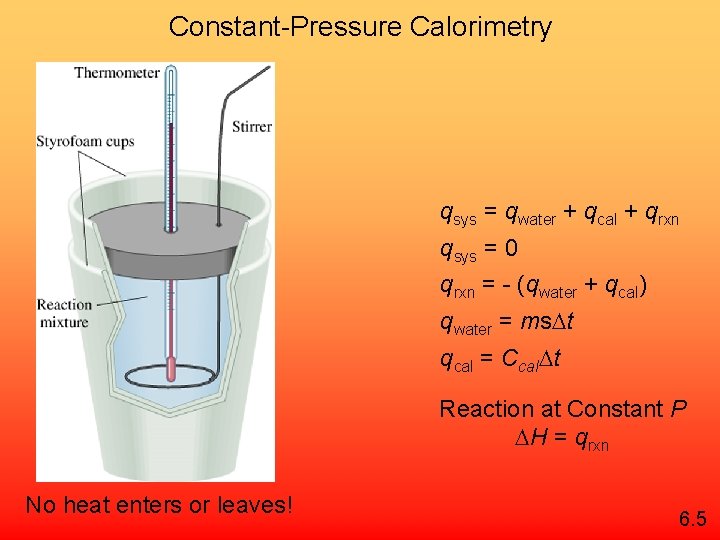
Constant-Pressure Calorimetry qsys = qwater + qcal + qrxn qsys = 0 qrxn = - (qwater + qcal) qwater = ms t qcal = Ccal t Reaction at Constant P H = qrxn No heat enters or leaves! 6. 5

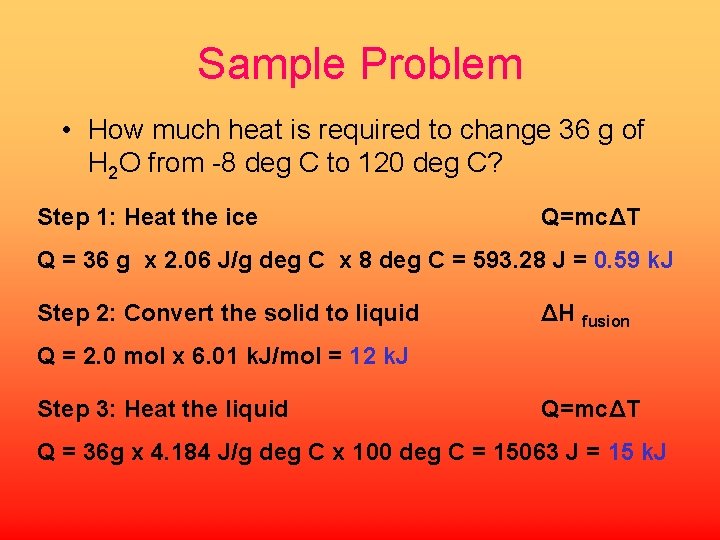
Sample Problem • How much heat is required to change 36 g of H 2 O from -8 deg C to 120 deg C? Step 1: Heat the ice Q=mcΔT Q = 36 g x 2. 06 J/g deg C x 8 deg C = 593. 28 J = 0. 59 k. J Step 2: Convert the solid to liquid ΔH fusion Q = 2. 0 mol x 6. 01 k. J/mol = 12 k. J Step 3: Heat the liquid Q=mcΔT Q = 36 g x 4. 184 J/g deg C x 100 deg C = 15063 J = 15 k. J

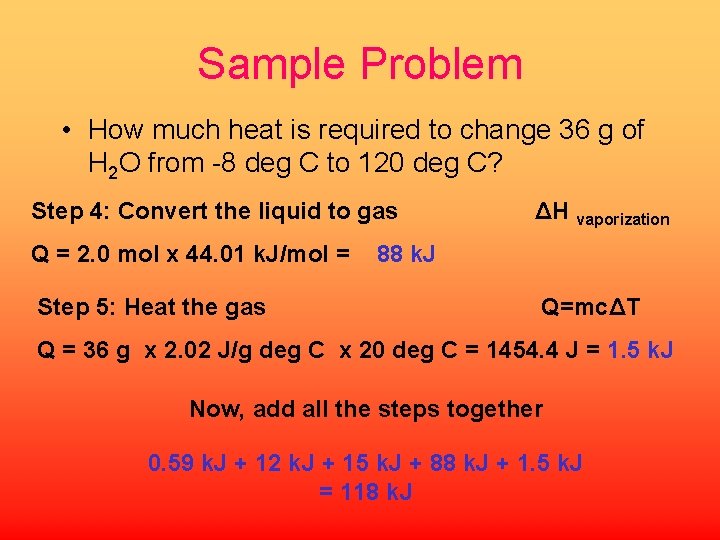
Sample Problem • How much heat is required to change 36 g of H 2 O from -8 deg C to 120 deg C? Step 4: Convert the liquid to gas Q = 2. 0 mol x 44. 01 k. J/mol = Step 5: Heat the gas ΔH vaporization 88 k. J Q=mcΔT Q = 36 g x 2. 02 J/g deg C x 20 deg C = 1454. 4 J = 1. 5 k. J Now, add all the steps together 0. 59 k. J + 12 k. J + 15 k. J + 88 k. J + 1. 5 k. J = 118 k. J