Phases of Matter Kinetic Theory All matter is








- Slides: 23

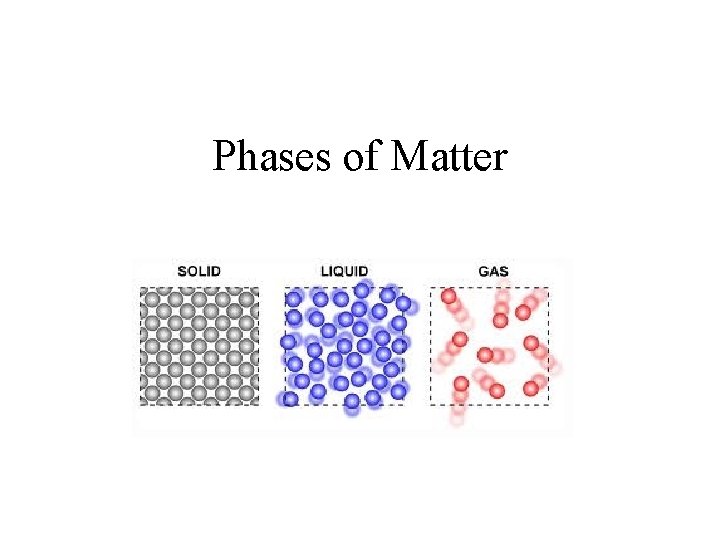
Phases of Matter

Kinetic Theory • All matter is made of atoms and molecules that act like tiny particles. • These tiny particles are always in motion. The higher the temp. , the faster the particles move. • At the same temp. , more massive (heavier) particles move slower than less massive (lighter) particles.

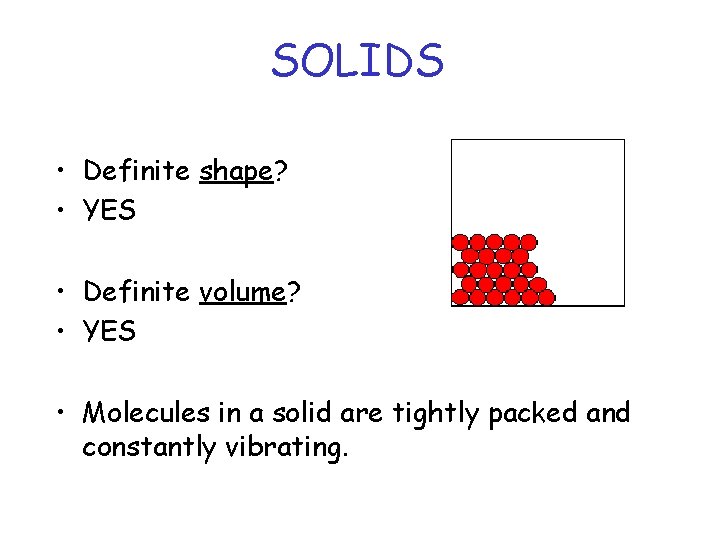
SOLIDS • Definite shape? • YES • Definite volume? • YES • Molecules in a solid are tightly packed and constantly vibrating.

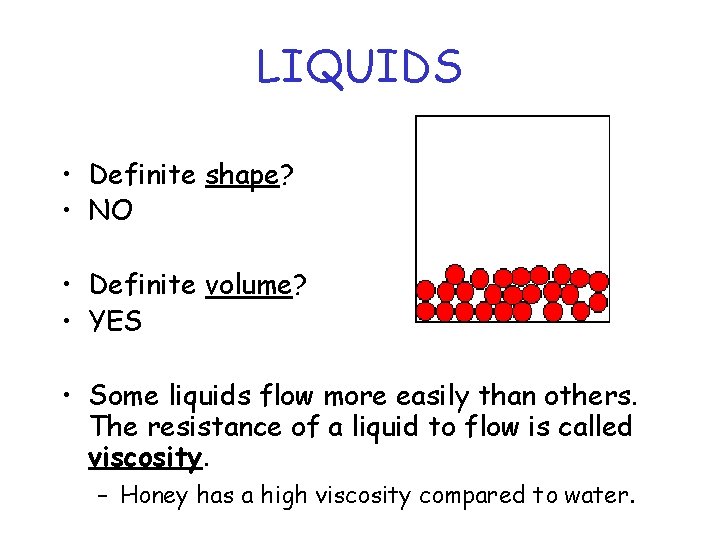
LIQUIDS • Definite shape? • NO • Definite volume? • YES • Some liquids flow more easily than others. The resistance of a liquid to flow is called viscosity. – Honey has a high viscosity compared to water.

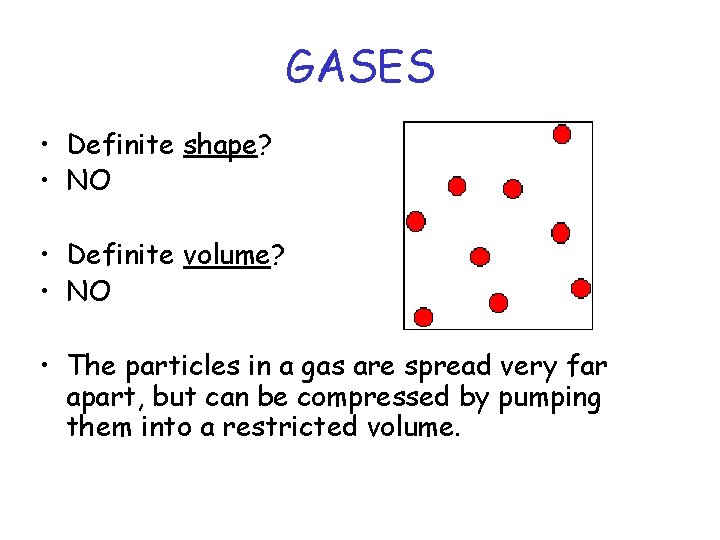
GASES • Definite shape? • NO • Definite volume? • NO • The particles in a gas are spread very far apart, but can be compressed by pumping them into a restricted volume.

Phase Changes • Changes in phase are examples of physical changes. • • • Melting: solid liquid Freezing: liquid solid Vaporization: liquid gas Condensation: gas liquid Sublimation: solid gas

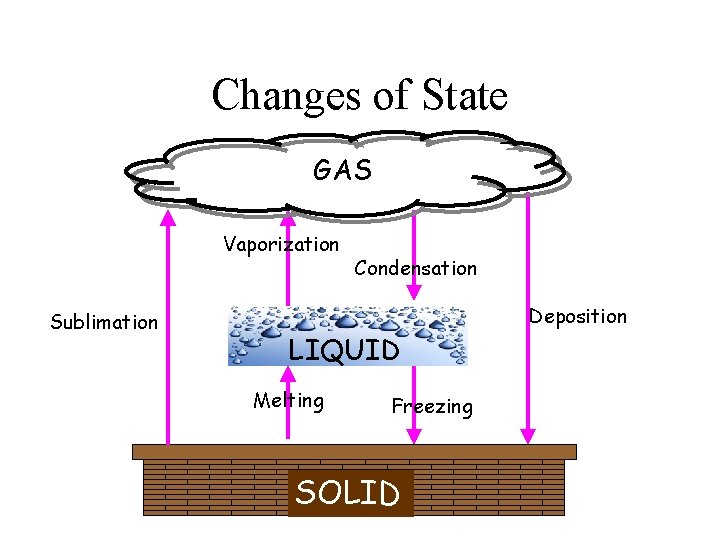
Changes of State GAS Vaporization Sublimation Condensation Deposition Melting LIQUID Melting Freezing SOLID

• ENERGY is the ability to change or move matter. • Energy is ABSORBED when substances melt or evaporate. – NOTE: our bodies cool down when our sweat evaporates. • Energy is RELEASED when substances freeze or condense.

Melting • The change of state from solid to liquid. • Energy (heat) is absorbed by the substance that is melting.

Freezing • The change of state from liquid to solid. Opposite of melting. Energy (heat) is released by the substance undergoing freezing.

• Evaporation The change of state at the surface of a liquid as it passes to a vapor. This results from the random motion of molecules that occasionally escape from the liquid surface. – Energy (heat) is absorbed by the liquid (Cooling of the liquid results) – Can happen at any temperature

Condensation • The change of state from gas to liquid. The opposite of evaporation. – Energy (heat) is released by the liquid (Warming of the liquid results)

Boiling • Change from state from a liquid to a gas. • Occurs throughout the liquid. – boiling point/temperature is determined by pressure – Energy (heat) is absorbed by the liquid.

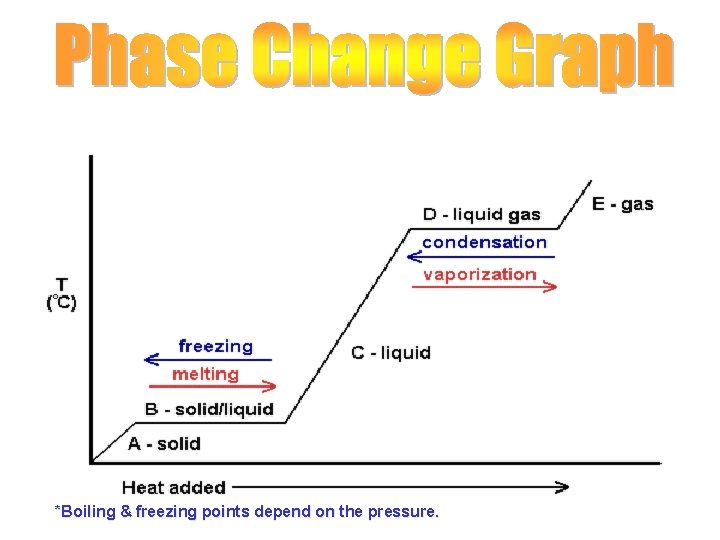
*Boiling & freezing points depend on the pressure.

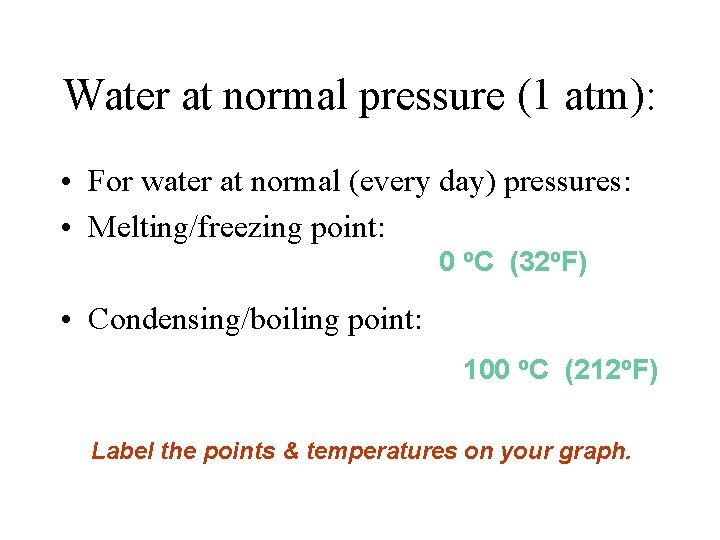
Water at normal pressure (1 atm): • For water at normal (every day) pressures: • Melting/freezing point: 0 o. C (32 o. F) • Condensing/boiling point: 100 o. C (212 o. F) Label the points & temperatures on your graph.

Change the pressure Change the Boiling Point

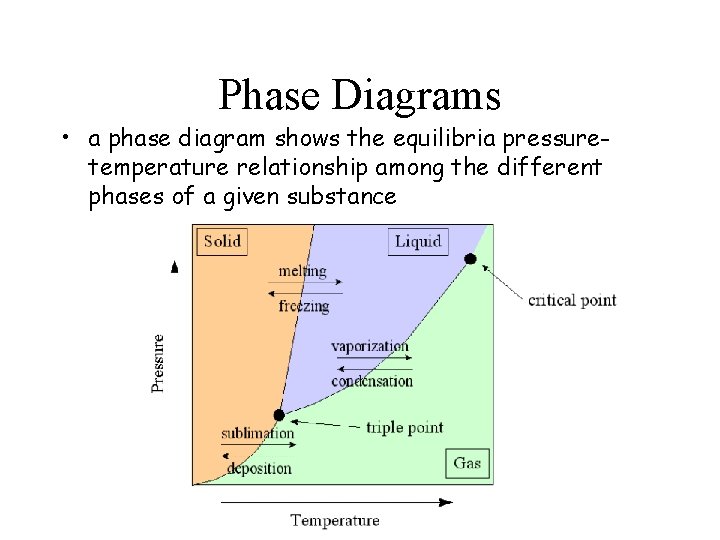
Phase Diagrams • a phase diagram shows the equilibria pressuretemperature relationship among the different phases of a given substance

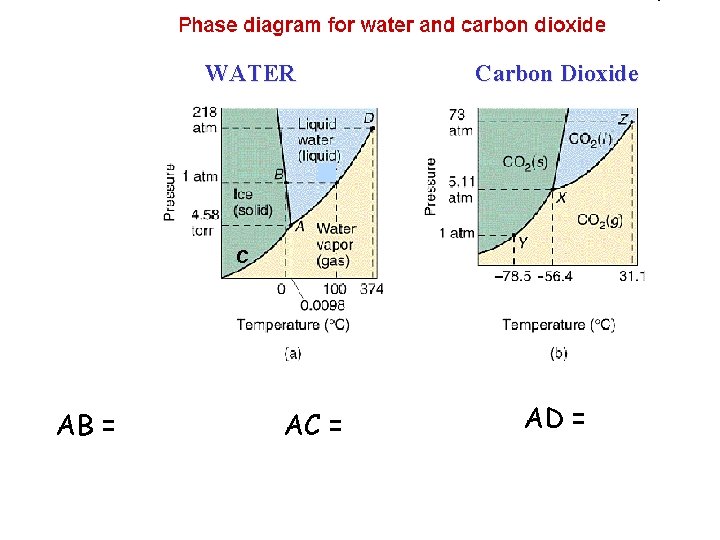
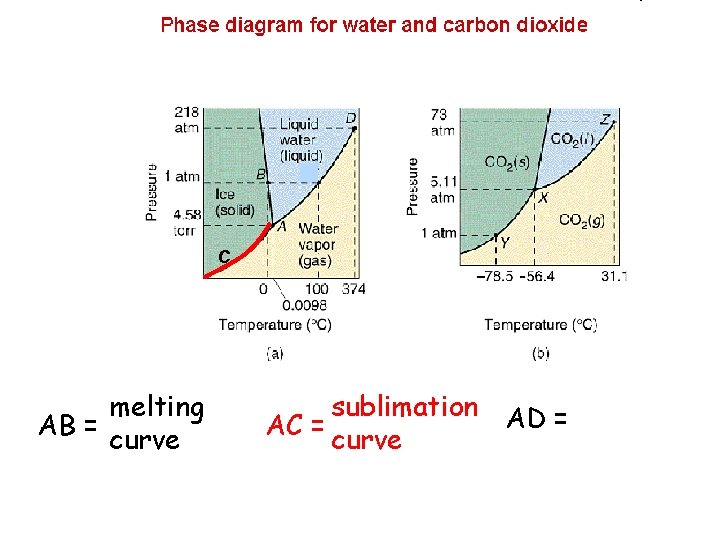
WATER Carbon Dioxide C AB = AC = AD =

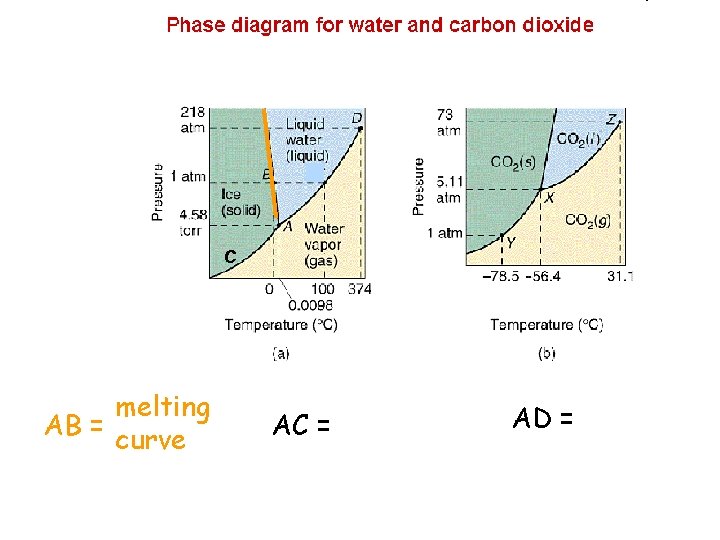
C melting AB = curve AC = AD =

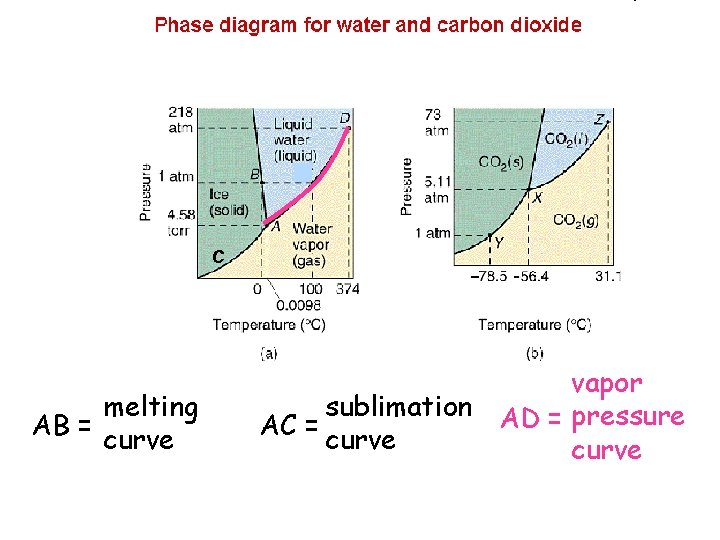
C melting AB = curve sublimation AD = AC = curve

C melting AB = curve vapor sublimation AD = pressure AC = curve

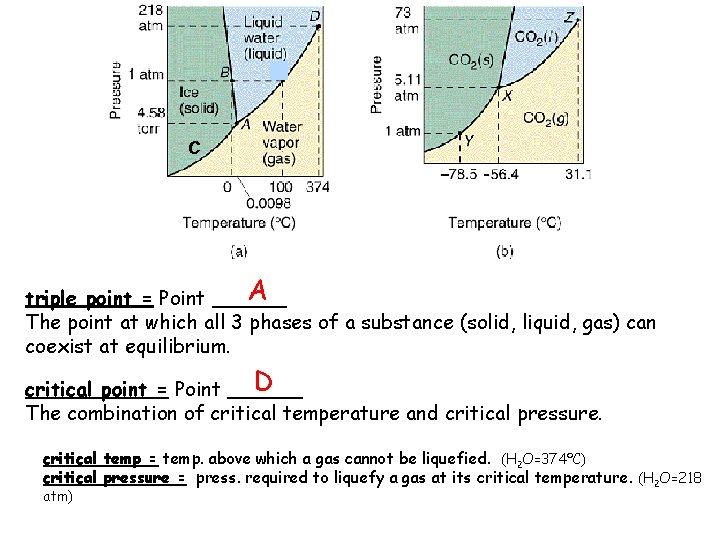
C A triple point = Point ______ The point at which all 3 phases of a substance (solid, liquid, gas) can coexist at equilibrium. D critical point = Point ______ The combination of critical temperature and critical pressure. critical temp = temp. above which a gas cannot be liquefied. (H 2 O=374ºC) critical pressure = press. required to liquefy a gas at its critical temperature. (H 2 O=218 atm)

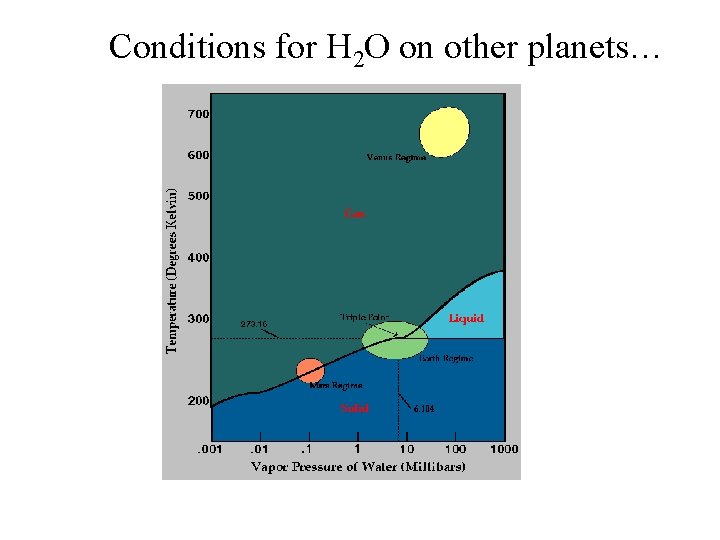
Conditions for H 2 O on other planets…
 The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Define kinetic theory of matter
Define kinetic theory of matter An explanation of how particles in matter behave
An explanation of how particles in matter behave Kinetic particle theory questions
Kinetic particle theory questions What is a kinetic theory of matter
What is a kinetic theory of matter Name
Name Four phases of matter
Four phases of matter 4 phases of matter
4 phases of matter Phases changes of matter
Phases changes of matter 3 phases of matter
3 phases of matter Why isn't it a good idea to classify matter by its phases
Why isn't it a good idea to classify matter by its phases Whats the study of matter and energy
Whats the study of matter and energy Phases of matter
Phases of matter Concept map states of matter
Concept map states of matter Phases of the moon
Phases of the moon Kinetic molecular theory of solid
Kinetic molecular theory of solid Kinetic molecular model of gases
Kinetic molecular model of gases The kinetic molecular theory
The kinetic molecular theory Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic molecular theory
Kinetic molecular theory Kinetic molecular theory def
Kinetic molecular theory def Timeline of kinetic molecular theory
Timeline of kinetic molecular theory