Calculations in Chemistry IGCSE Chemistry Mole concept Relative







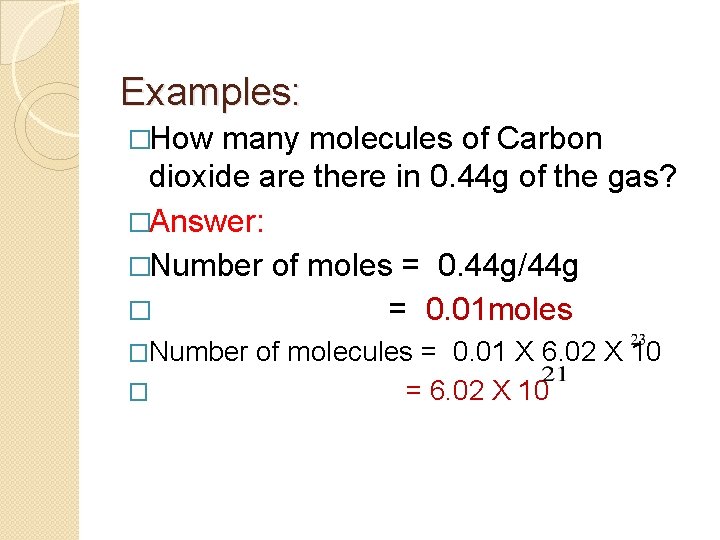





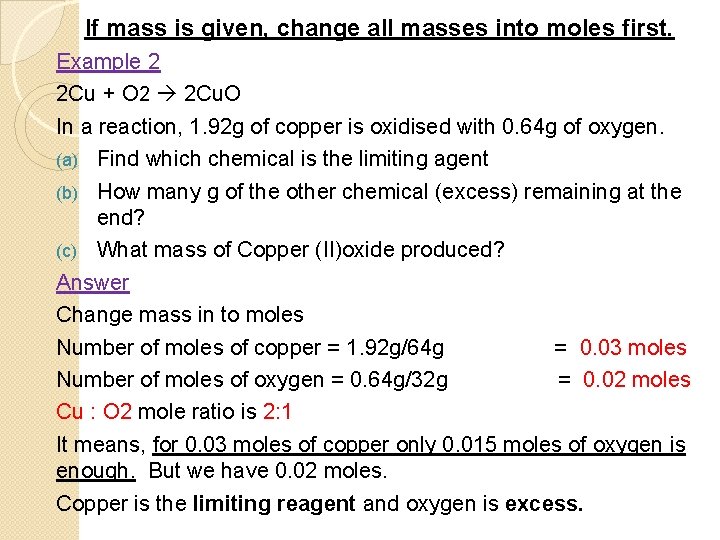



- Slides: 29

Calculations in Chemistry IGCSE Chemistry

�Mole concept

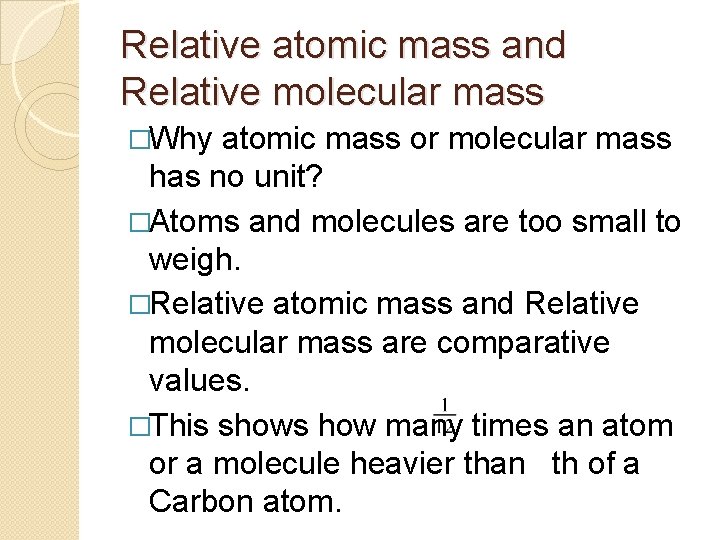
Relative atomic mass and Relative molecular mass �Why atomic mass or molecular mass has no unit? �Atoms and molecules are too small to weigh. �Relative atomic mass and Relative molecular mass are comparative values. �This shows how many times an atom or a molecule heavier than th of a Carbon atom.

The mole…. �Atomic mass or molecular mass with g after the number is called a mole. �Example: �Atomic mass of Oxygen is 16. � 16 g of oxygen(O) is 1 mole of oxygen atoms. �Molecular mass of Oxygen O 2 is 32. � 32 g of Oxygen (O 2) is 1 mole of oxygen molecules. �Molecular mass of water is 18. � 18 g of water is called 1 mole of water.

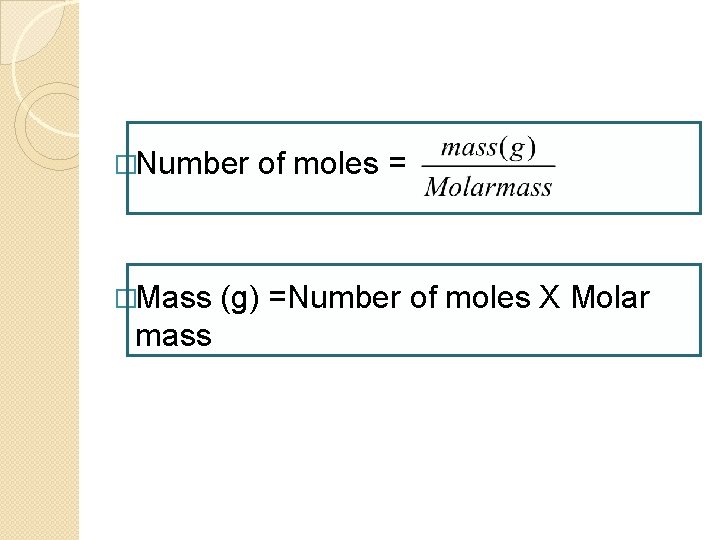
�Number �Mass mass of moles = (g) =Number of moles X Molar

Questions � 1. How many moles are there in 4 g of Sodium hydroxide? �Number of moles = = 0. 10 moles � 2. Find the number of moles present in 1. 28 g of oxygen molecules. �Number of moles = � � = 0. 04 moles

� 3. What is the mass of 0. 01 moles of sodium hydroxide? �Mass = 0. 01 X 40 g � = 0. 40 g � 4. How many grams 1. 5 moles of sulphuric acid weighs? �Mass = 1. 5 X 98 g � = 147 g

Try the following questions: � 1. What is the mass of 0. 02 moles of carbon dioxide gas? � 0. 88 g � 2. How many moles are present in 1 g of calcium carbonate, Ca. CO 3? � 0. 01 moles � 3. Find the number of moles present in 3 g of potassium hydroxide, KOH. � 0. 054 moles � 4. Calculate the mass of 100 moles of copper oxide, Cu. O � 8000 g � 5. 5. 2 g of sodium hydroxide, Na. OH or 3 g of potassium hydroxide, KOH contains more number of moles? Show your working clearly. � Na. OH = 0. 13 moles KOH = 0. 054 moles. So Na. OH

Avogadro constant �One mole of any substance contains 6. 02 X 10 atoms or molecules. � 6. 02 X 10 is a constant and is called Avogadro constant or Avogadro Number. �In other words, Avogadro Number is the number of atoms or number of molecules present in ONE mole of a substance. �Avogadro constant is used to find

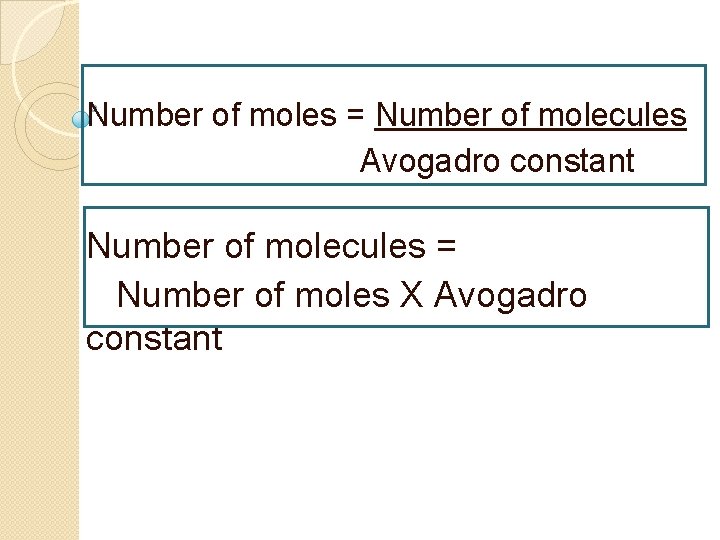
Number of moles = Number of molecules Avogadro constant Number of molecules = Number of moles X Avogadro constant

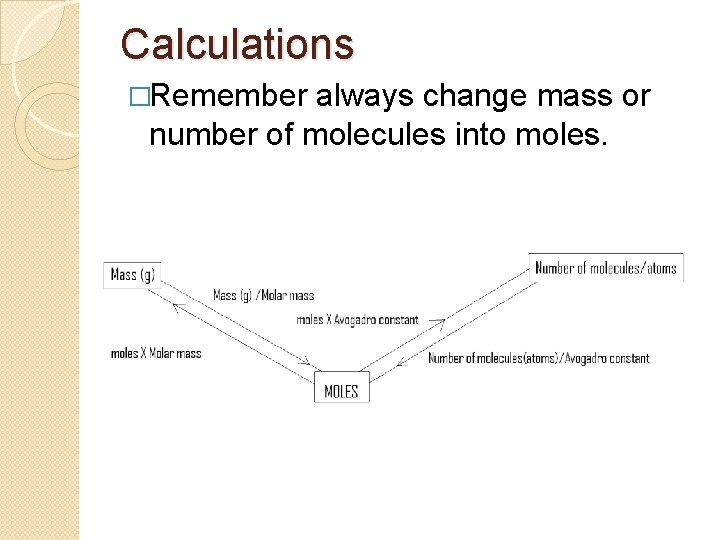
Calculations �Remember always change mass or number of molecules into moles.
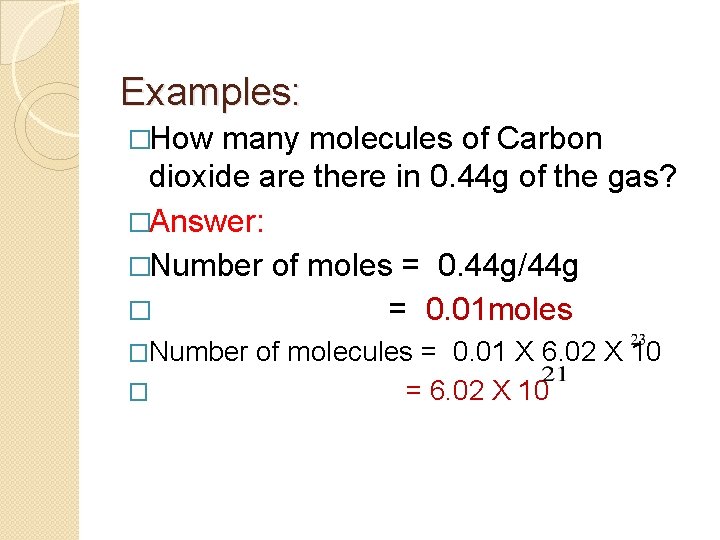
Examples: �How many molecules of Carbon dioxide are there in 0. 44 g of the gas? �Answer: �Number of moles = 0. 44 g/44 g � = 0. 01 moles �Number � of molecules = 0. 01 X 6. 02 X 10 = 6. 02 X 10

Calculate how many oxygen atoms are present in 1. 96 g of sulphuric acid? �Answer: �Moles of sulphuric acid = 1. 96 g/98 g � = 0. 02 moles �Number of molecules of sulphuric acid � = 0. 02 X 6. 02 X 10 � =1. 20 X 10 �Each sulphuric acid contains 4 oxygen atoms. �So number of oxygen atoms 0. 02 moles of H 2 SO 4 is

Mole ratio �When chemical reactions take place, the reactants and products are in a simple ratio (mole ratio) �For example, Zinc and hydrochloric acid react according to the following reaction: � Zinc gas + Hydrochloric acid Zinc chloride + Hydrogen � Zn � It + 2 HCl Zn. Cl 2 + H 2 means, zinc, Hydrochloric acid, Zinc chloride and Hydrogen moles are in the ratio 1: 2: 1: 1 � One mole of Zinc reacts with 2 moles of Hydrochloric acid to produce, One mole of Zinc chloride and 1 mole of Hydrogen gas. � The reactants and products are always in a ratio by moles and not by mass.

Find how many grams of copper oxide can be made from 3. 2 g of copper. �Answer: � 2 Cu + O 2 2 Cu. O �The balanced equation shows that two moles of copper reacts with 1 mole of oxygen to form 2 moles of copper(II) oxide. �So change 3. 2 g to moles �Moles of copper = 3. 2 g/64 g =0. 05 moles �Copper : Copper oxide is 1: 1 ratio. �So moles of copper(II) oxide = 0. 05 moles �Mass (g) = 0. 05 X 80 g � = 4 g

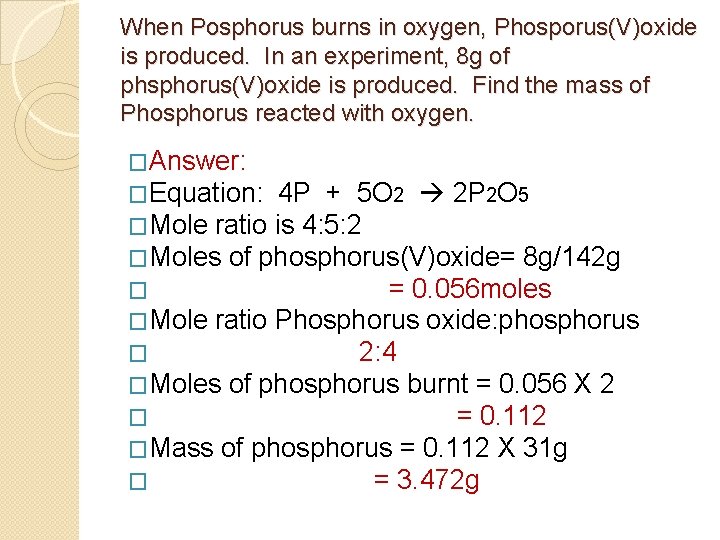
When Posphorus burns in oxygen, Phosporus(V)oxide is produced. In an experiment, 8 g of phsphorus(V)oxide is produced. Find the mass of Phosphorus reacted with oxygen. �Answer: �Equation: 4 P + 5 O 2 2 P 2 O 5 �Mole ratio is 4: 5: 2 �Moles of phosphorus(V)oxide= 8 g/142 g � = 0. 056 moles �Mole ratio Phosphorus oxide: phosphorus � 2: 4 �Moles of phosphorus burnt = 0. 056 X 2 � = 0. 112 �Mass of phosphorus = 0. 112 X 31 g � = 3. 472 g

Limiting reagent and excess �When there is a chemical reaction, the reactant which is used up completely is the limiting reagent. �The chemical which is left some unreacted at the end is called excess. �When the limiting reagent is finished, the reaction stops. �All mole ratios should be done using the limiting reagent.

�Example 1 � 3 Mg + Al 2 O 3 3 Mg. O + 2 Al �If 2 moles of Magnesium is used with 1 moles of aluminium oxide, which is the limiting agent? Which chemical is in excess? How many moles in excess? �Mole ratio of Mg and Al 2 O 3 is 3: 1 �It means for 2 moles of Mg, 0. 67 moles of Al 2 O 3 is enough. But we have 1 mole of Al 2 O 3. So Al 2 O 3 is excess and Mg is the limiting reagent. �Moles of Al 2 O 3 (excess) left after reaction is
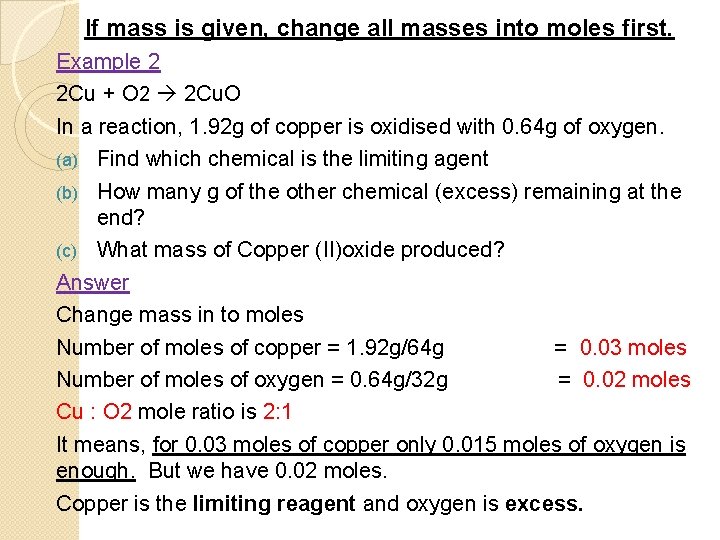
If mass is given, change all masses into moles first. Example 2 2 Cu + O 2 2 Cu. O In a reaction, 1. 92 g of copper is oxidised with 0. 64 g of oxygen. (a) Find which chemical is the limiting agent (b) How many g of the other chemical (excess) remaining at the end? (c) What mass of Copper (II)oxide produced? Answer Change mass in to moles Number of moles of copper = 1. 92 g/64 g = 0. 03 moles Number of moles of oxygen = 0. 64 g/32 g = 0. 02 moles Cu : O 2 mole ratio is 2: 1 It means, for 0. 03 moles of copper only 0. 015 moles of oxygen is enough. But we have 0. 02 moles. Copper is the limiting reagent and oxygen is excess.

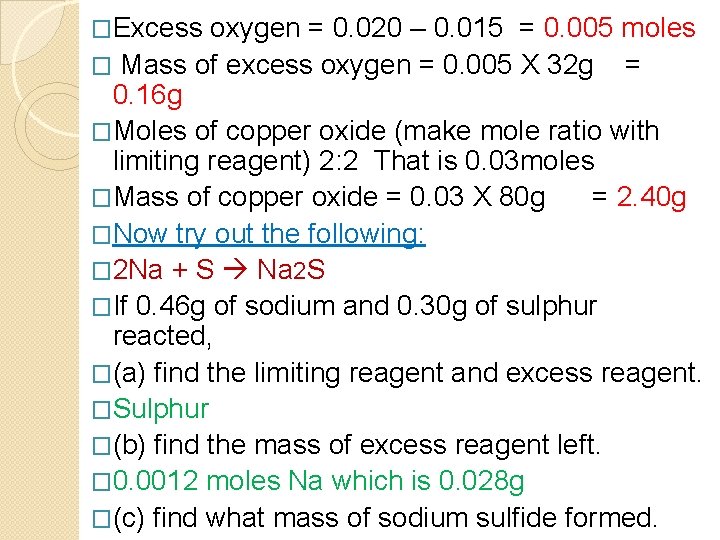
�Excess oxygen = 0. 020 – 0. 015 = 0. 005 moles � Mass of excess oxygen = 0. 005 X 32 g = 0. 16 g �Moles of copper oxide (make mole ratio with limiting reagent) 2: 2 That is 0. 03 moles �Mass of copper oxide = 0. 03 X 80 g = 2. 40 g �Now try out the following: � 2 Na + S Na 2 S �If 0. 46 g of sodium and 0. 30 g of sulphur reacted, �(a) find the limiting reagent and excess reagent. �Sulphur �(b) find the mass of excess reagent left. � 0. 0012 moles Na which is 0. 028 g �(c) find what mass of sodium sulfide formed.

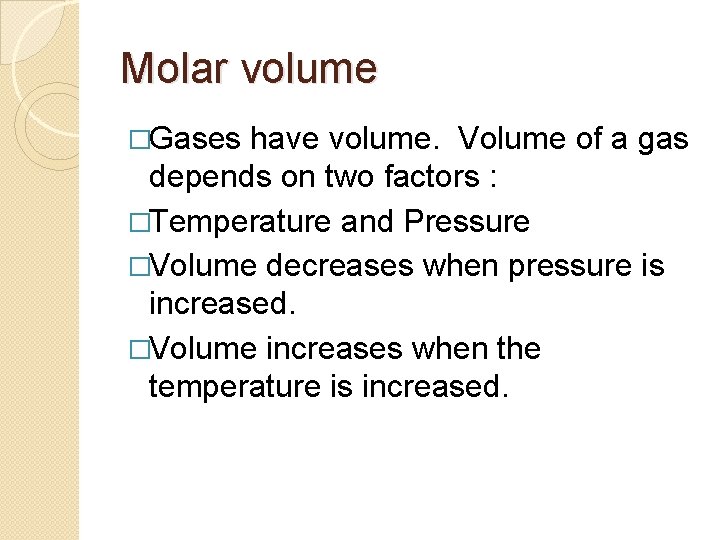
Molar volume �Gases have volume. Volume of a gas depends on two factors : �Temperature and Pressure �Volume decreases when pressure is increased. �Volume increases when the temperature is increased.

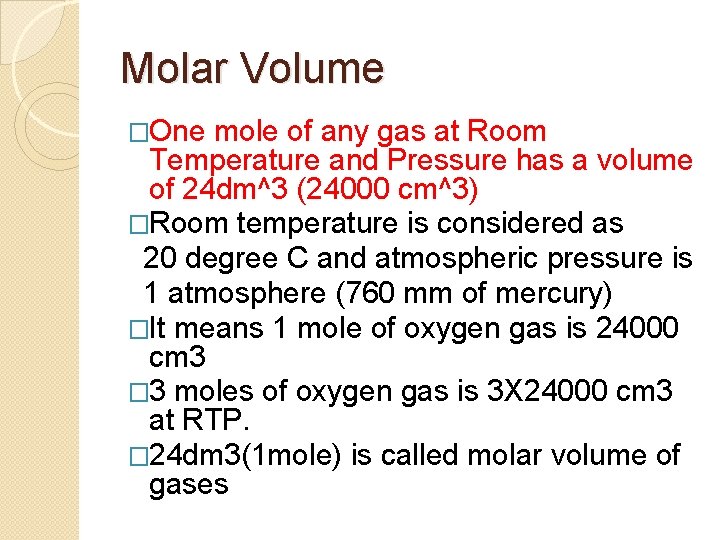
Molar Volume �One mole of any gas at Room Temperature and Pressure has a volume of 24 dm^3 (24000 cm^3) �Room temperature is considered as 20 degree C and atmospheric pressure is 1 atmosphere (760 mm of mercury) �It means 1 mole of oxygen gas is 24000 cm 3 � 3 moles of oxygen gas is 3 X 24000 cm 3 at RTP. � 24 dm 3(1 mole) is called molar volume of gases

�Moles X molar volume = volume of a gas �Moles = volume of a gas/molar volume Remember to convert volume or mass to moles first. Example 1 What is the volume of 1. 1 g of carbon dioxide gas at RTP? First convert mass in to moles Moles of CO 2 = 1. 1 g/44 g = 0. 025 moles Volume = moles X molar volume = 0. 025 X 24000 cm 3 = 600 cm 3

�Example 2 �Find out the mass of 8000 cm 3 of sulphur dioxide gas, SO 2 at RTP. �First change the volume into moles. �Moles = Volume/molar volume � 8000 cm 3/24000 cm 3 � = 0. 33 moles �Now change the moles in to mass �Mass = moles X molar mass � 0. 33 X 64 g = 21. 12 g

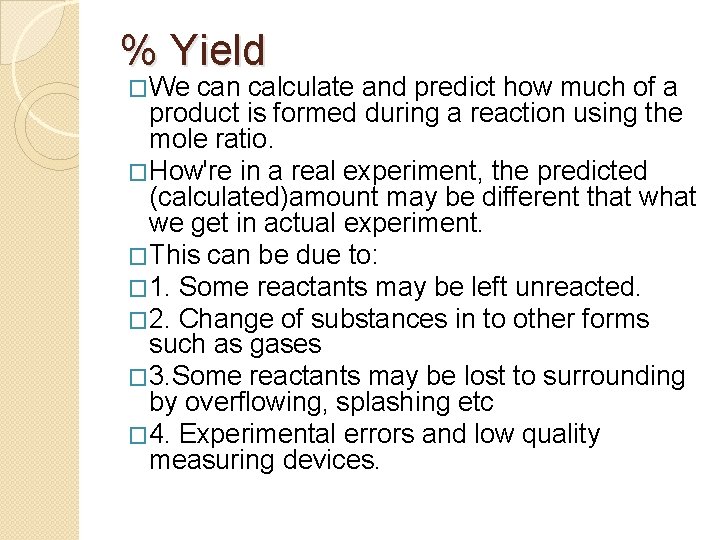
% Yield �We can calculate and predict how much of a product is formed during a reaction using the mole ratio. �How're in a real experiment, the predicted (calculated)amount may be different that we get in actual experiment. �This can be due to: � 1. Some reactants may be left unreacted. � 2. Change of substances in to other forms such as gases � 3. Some reactants may be lost to surrounding by overflowing, splashing etc � 4. Experimental errors and low quality measuring devices.

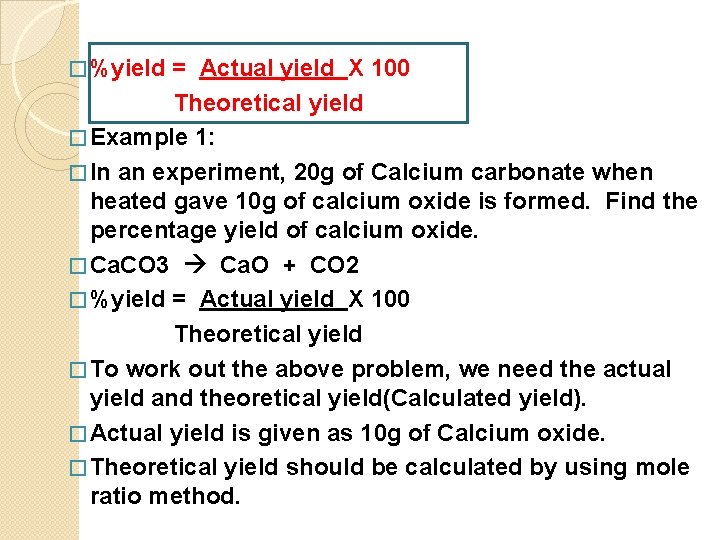
� %yield = Actual yield X 100 Theoretical yield � Example 1: � In an experiment, 20 g of Calcium carbonate when heated gave 10 g of calcium oxide is formed. Find the percentage yield of calcium oxide. � Ca. CO 3 Ca. O + CO 2 � %yield = Actual yield X 100 Theoretical yield � To work out the above problem, we need the actual yield and theoretical yield(Calculated yield). � Actual yield is given as 10 g of Calcium oxide. � Theoretical yield should be calculated by using mole ratio method.

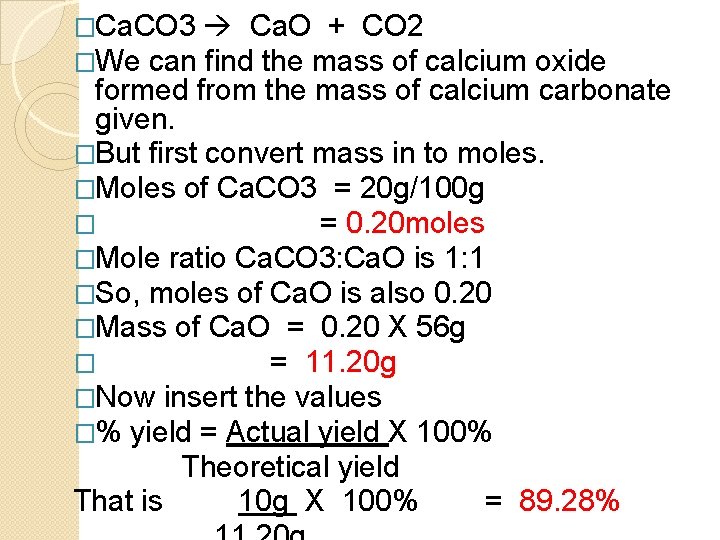
�Ca. CO 3 Ca. O + CO 2 �We can find the mass of calcium oxide formed from the mass of calcium carbonate given. �But first convert mass in to moles. �Moles of Ca. CO 3 = 20 g/100 g � = 0. 20 moles �Mole ratio Ca. CO 3: Ca. O is 1: 1 �So, moles of Ca. O is also 0. 20 �Mass of Ca. O = 0. 20 X 56 g � = 11. 20 g �Now insert the values �% yield = Actual yield X 100% Theoretical yield That is 10 g X 100% = 89. 28%

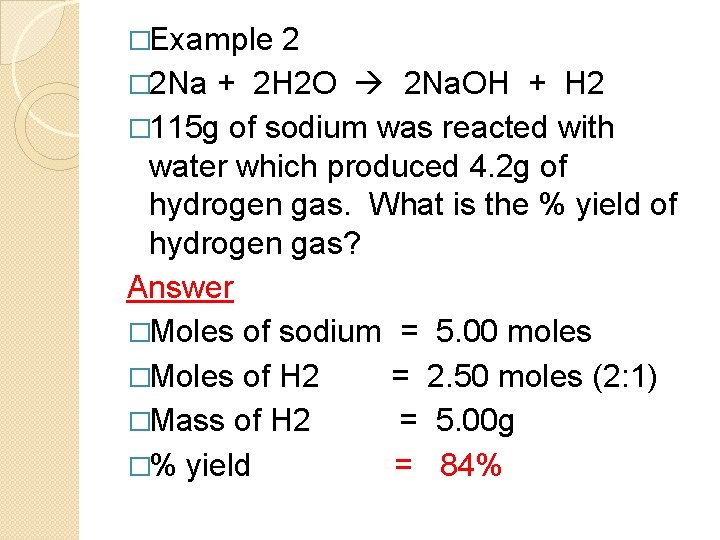
�Example 2 � 2 Na + 2 H 2 O 2 Na. OH + H 2 � 115 g of sodium was reacted with water which produced 4. 2 g of hydrogen gas. What is the % yield of hydrogen gas? Answer �Moles of sodium = 5. 00 moles �Moles of H 2 = 2. 50 moles (2: 1) �Mass of H 2 = 5. 00 g �% yield = 84%

End of part 1
 Types of connections in steel structures
Types of connections in steel structures 22,4 l/mol
22,4 l/mol Gram to gram conversion
Gram to gram conversion Mole-mole factor
Mole-mole factor Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Phosphorus and oxygen equation
Phosphorus and oxygen equation Stoichiometry mole-mole
Stoichiometry mole-mole Convert mass to moles
Convert mass to moles Mole-mass-volume relationships
Mole-mass-volume relationships Igcse chemistry syllabus
Igcse chemistry syllabus Board works chemistry
Board works chemistry Serum osmolality formula
Serum osmolality formula Moles in chemistry formula
Moles in chemistry formula Mole concept
Mole concept Mole concept
Mole concept What's a mole chemistry
What's a mole chemistry No of moles formula
No of moles formula Mole hill chemistry
Mole hill chemistry Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Mole bridge
Mole bridge Mole bridge chemistry
Mole bridge chemistry Stoichiometry cookie lab
Stoichiometry cookie lab Mole island diagram
Mole island diagram Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Let's make some cookies
Let's make some cookies Grams to moles formula
Grams to moles formula Relative clauses and relative pronouns stage 15
Relative clauses and relative pronouns stage 15 Marginal relative frequency
Marginal relative frequency The person who phoned me last night is my teacher.
The person who phoned me last night is my teacher. Relative adverbs and pronouns
Relative adverbs and pronouns