Alkaloids Derived from Phenylalanine and Tyrosine hsan ALI































- Slides: 62

Alkaloids Derived from Phenylalanine and Tyrosine İhsan ÇALIŞ References R. Hänsel&O. Sticher, Pharmakognosie, Phytopharmazie, Springer, Heidelberg 2007 J. Bruneton, Pharmacognosy: Phytochemistry - Medicinal Plants, Lavosier, Paris 1999, 2009

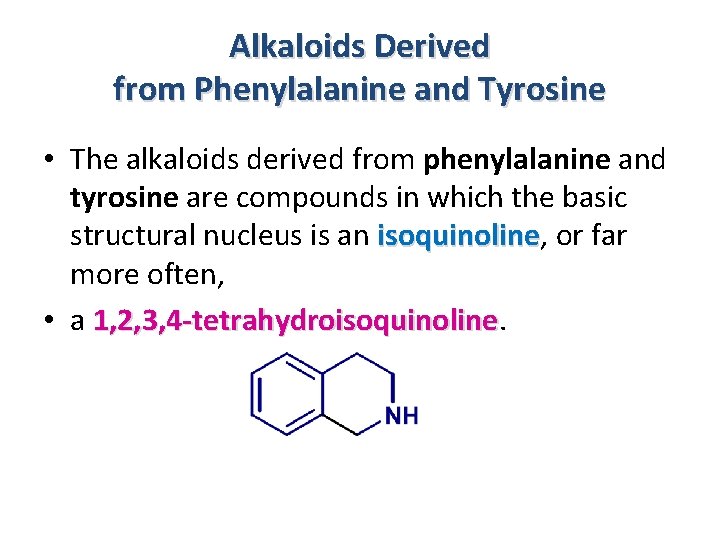
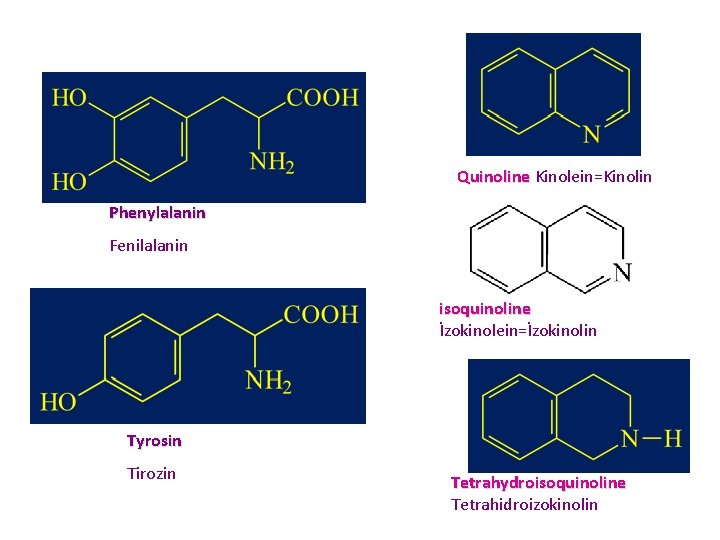
Alkaloids Derived from Phenylalanine and Tyrosine • GENERALITIES • A very large number of alkaloid structures arise from the metabolism of aromatic amino acids (phenylalanine, tyrosine). tyrosine • These are always isoquinoline alkaloids.

Alkaloids Derived from Phenylalanine and Tyrosine • The alkaloids derived from phenylalanine and tyrosine are compounds in which the basic structural nucleus is an isoquinoline, or far isoquinoline more often, • a 1, 2, 3, 4 -tetrahydroisoquinoline

Quinoline Kinolein=Kinolin Phenylalanin Fenilalanin isoquinoline İzokinolein=İzokinolin Tyrosin Tirozin Tetrahydroisoquinoline Tetrahidroizokinolin

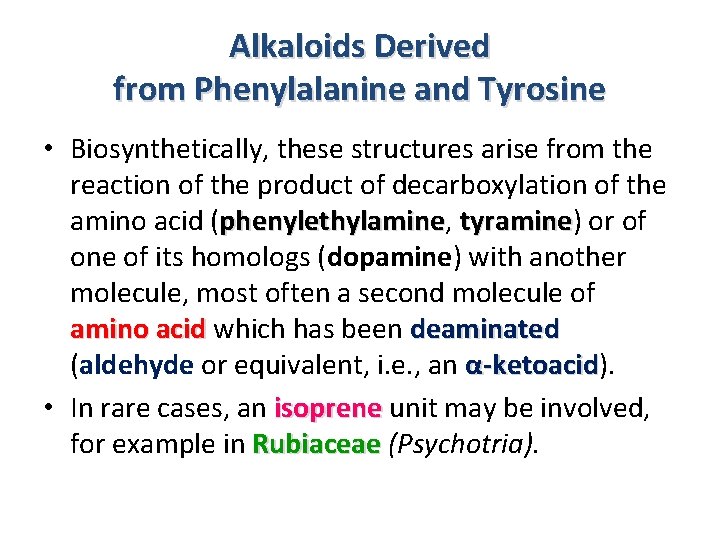
Alkaloids Derived from Phenylalanine and Tyrosine • Biosynthetically, these structures arise from the reaction of the product of decarboxylation of the amino acid (phenylethylamine, phenylethylamine tyramine) or of tyramine of its homologs (dopamine) with another molecule, most often a second molecule of amino acid which has been deaminated (aldehyde or equivalent, i. e. , an α-ketoacid). -ketoacid • In rare cases, an isoprene unit may be involved, for example in Rubiaceae (Psychotria).

Alkaloids Derived from Phenylalanine and Tyrosine • Besides the phenethylamines, they are classified under the five main groups as a function of the nature of the precursor(s) which react(s) with the aromatic amino acid to form the final structure.

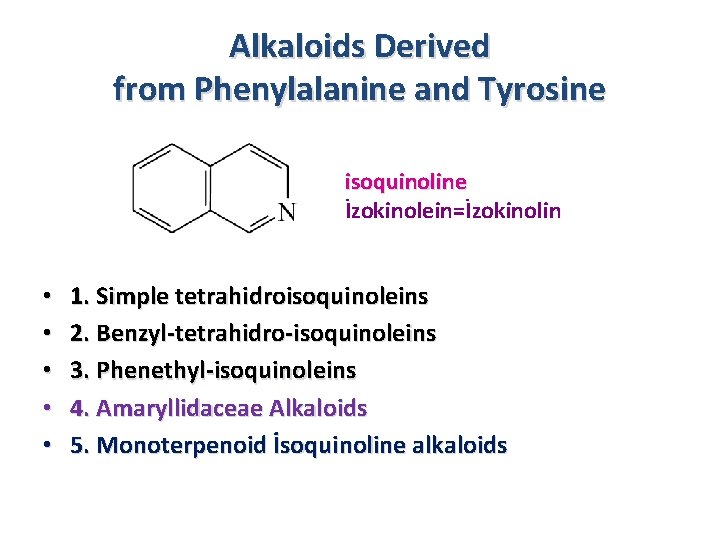
Alkaloids Derived from Phenylalanine and Tyrosine isoquinoline İzokinolein=İzokinolin • • • 1. Simple tetrahidroisoquinoleins 2. Benzyl-tetrahidro-isoquinoleins 3. Phenethyl-isoquinoleins 4. Amaryllidaceae Alkaloids 5. Monoterpenoid İsoquinoline alkaloids

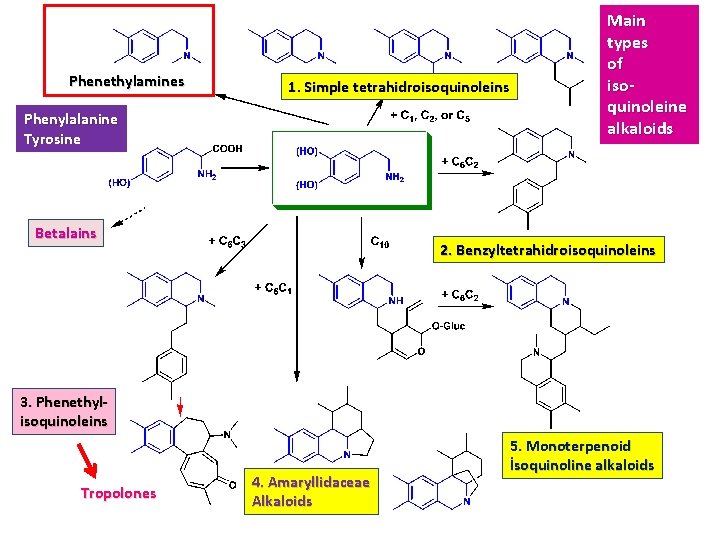
Phenethylamines 1. Simple tetrahidroisoquinoleins Phenylalanine Tyrosine Betalains Main types of isoquinoleine alkaloids 2. Benzyltetrahidroisoquinoleins 3. Phenethylisoquinoleins Tropolones 4. Amaryllidaceae Alkaloids 5. Monoterpenoid İsoquinoline alkaloids

Alkaloids Derived from Phenylalanine and Tyrosine Simple Tetrahydroisoquinoline Alks. Caryophyllales: Cactaceae, Chenopodiaceae Benzyltetrahydroisoquinoline Alks. Magnoliales, Laurales, or Papaverales (Annonaceae, Magnoliaceae, Lauraceae, Monimiaceae, Papaveraceae, Fumariaceae). Ranunculales (Berberidaceae, Menispermaceae, Ranunculaceae), (Euphorbiaceae, Fabaceae). Phenethylisoquinoline Alks. Liliaceae (Colchicum) Alkaloids of the Amaryllidaceae Clivia, Crinum, Galanthus, Haemanthus, Leucojum, Sternbergia Monoterpenoid Isoquinoline Alks. Rubiaceae

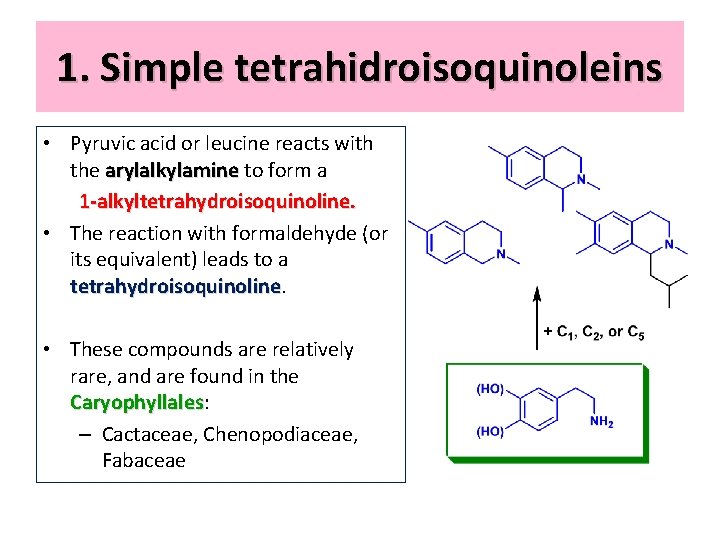
1. Simple tetrahidroisoquinoleins • Pyruvic acid or leucine reacts with the arylalkylamine to form a arylalkylamine 1 -alkyltetrahydroisoquinoline. • The reaction with formaldehyde (or its equivalent) leads to a tetrahydroisoquinoline • These compounds are relatively rare, and are found in the Caryophyllales: Caryophyllales – Cactaceae, Chenopodiaceae, Fabaceae

2. Benzyltetrahydroisoquinoline Alkaloids • Characterized by a C 6 C 2 -N-C 2 C 6 nucleus, this is the most important subgroup from the standpoint of size, and also structural variety and pharmacological potential. • The basic nucleus arises from The basic nucleus the reaction of the arylalkylamine with a second amino acid, tyrosine

2. Benzyltetrahydroisoquinoline Alkaloids • The different alkaloids in this group are characteristic of a certain number of families of the orders – Magnoliales, Laurales, or – Papaverales • Annonaceae, Magnoliaceae, Lauraceae, Monimiaceae, Papaveraceae, Fumariaceae – Ranunculales • Berberidaceae, Menispermaceae, Ranunculaceae

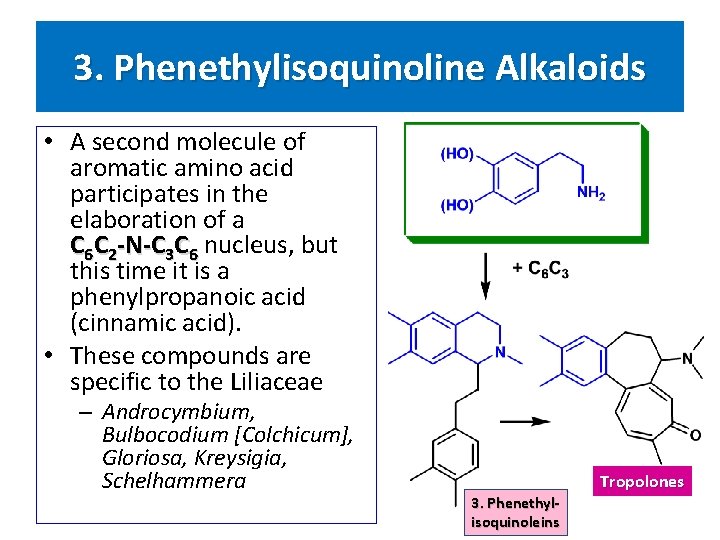
3. Phenethylisoquinoline Alkaloids • A second molecule of aromatic amino acid participates in the elaboration of a C 6 C 2 -N-C 3 C 6 nucleus, but this time it is a phenylpropanoic acid (cinnamic acid). • These compounds are specific to the Liliaceae – Androcymbium, Bulbocodium [Colchicum], Gloriosa, Kreysigia, Schelhammera 3. Phenethylisoquinoleins Tropolones

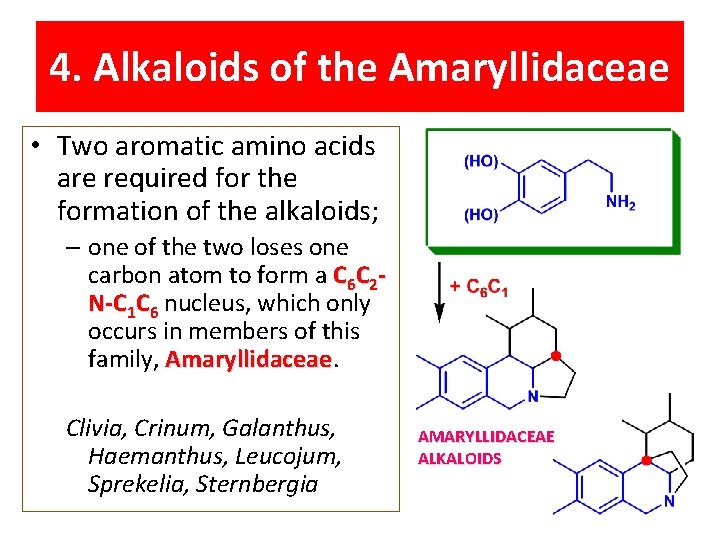
4. Alkaloids of the Amaryllidaceae • Two aromatic amino acids are required for the formation of the alkaloids; – one of the two loses one carbon atom to form a C 6 C 2 N-C 1 C 6 nucleus, which only occurs in members of this family, Amaryllidaceae Clivia, Crinum, Galanthus, Haemanthus, Leucojum, Sprekelia, Sternbergia AMARYLLIDACEAE ALKALOIDS

Alkaloids AMARYLLIDACEAE Activity Studies: • Cholinesterase inhibitory (acetylcholinesterase and butyrylcholinesterase) and tyrosinase inhibitory, activities Galanthus, Pancratium maritumum, Sternbergia species

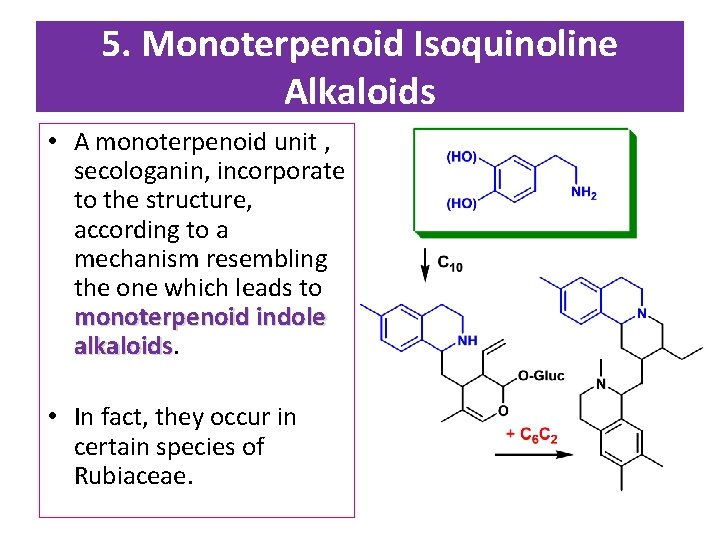
5. Monoterpenoid Isoquinoline Alkaloids • A monoterpenoid unit , secologanin, incorporate to the structure, according to a mechanism resembling the one which leads to monoterpenoid indole alkaloids • In fact, they occur in certain species of Rubiaceae.

Phenethylamines • Phenethylamine-containing Drugs – Ephedras: Ephedras • Ephedra spp. , Ephedraceae – Khat: Khat • Catha edulis Forsk. , Celastraceae

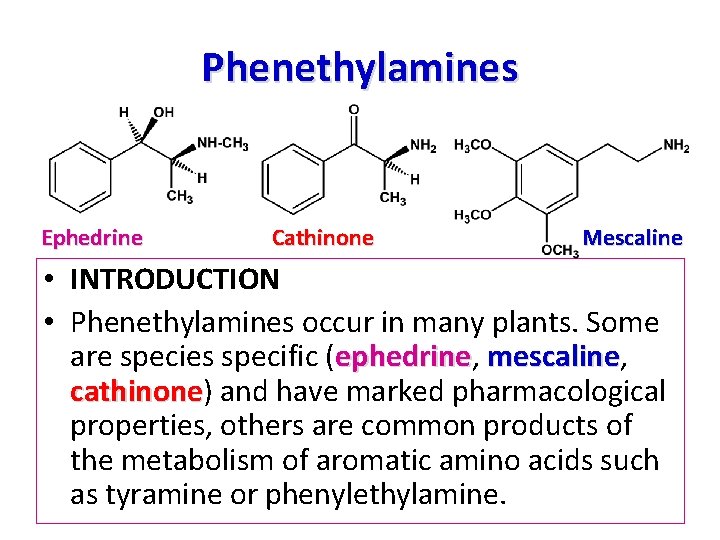
Phenethylamines Ephedrine Cathinone Mescaline • INTRODUCTION • Phenethylamines occur in many plants. Some are species specific (ephedrine, ephedrine mescaline, mescaline cathinone) and have marked pharmacological cathinone properties, others are common products of the metabolism of aromatic amino acids such as tyramine or phenylethylamine.

Phenethylamines • INTRODUCTION • Although the concentration of these decarboxylation products in edible or medicinal plants is too low to induce harmful effects, it is sometimes sufficient to play a role in the onset of an attack of migraine. • The effects of these amines, particularly tyramine, can tyramine become serious in patients treated with MAO inhibitors: inhibitors tyramine is no longer metabolized in the intestine and liver, and a risk of hypertensive crisis ensues. • Therefore, it is necessary to monitor the consumption of certain drugs by these patients (e. g. , Genista spec. : broom flowers), as well as certain vegetables ( avocado, cabbage, flowers cucumber, spinach) and certain other foods ( cheese). spinach cheese

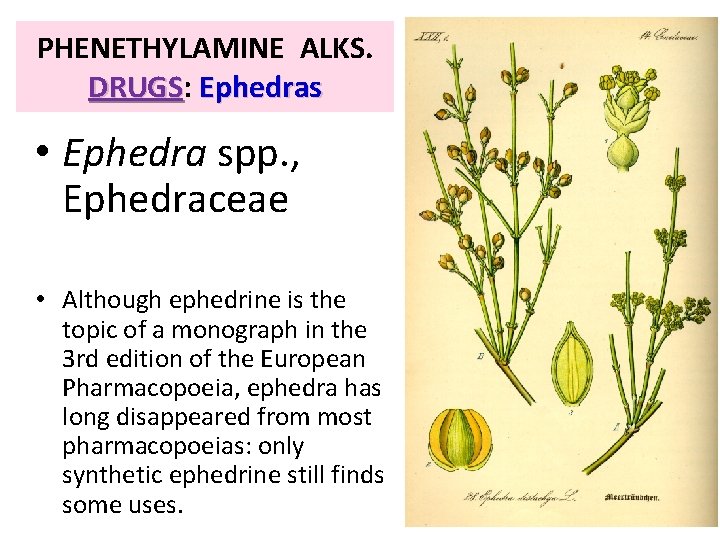
PHENETHYLAMINE ALKS. DRUGS: DRUGS Ephedras • Ephedra spp. , Ephedraceae • Although ephedrine is the topic of a monograph in the 3 rd edition of the European Pharmacopoeia, ephedra has long disappeared from most pharmacopoeias: only synthetic ephedrine still finds some uses.

EPHEDRAS Ephedra spp. , Ephedraceae • The Plants. Ephedras are dioecious subshrubs with the same habit as horsetails, with slender, angular, and striated branches, and with leaves reduced to membranous scales. • The female flowers are reduced to the ovule and surrounded by bracts that are red and fleshy at maturity. The male flowers are grouped in yellowish catkins.

EPHEDRAS Ephedra spp. , Ephedraceae • The species that contain substantial quantities of alkaloids are mostly Asian: – E. equisetina & E. sinica Stapf, from China, – E. intermedia & E. gerardiana, from India and Pakistan. • About ten species are found in North America, for example Mormon tea, tea E. nevadensis • Ephedras are seldom found in Europe: – E. major, E. procera, E. campylopoda, or E. distachya of the Atlantic coast. • Most of these species, except E. major, like the North American species, are thought to contain alkaloids at a negligible concentration or no alkaloids.

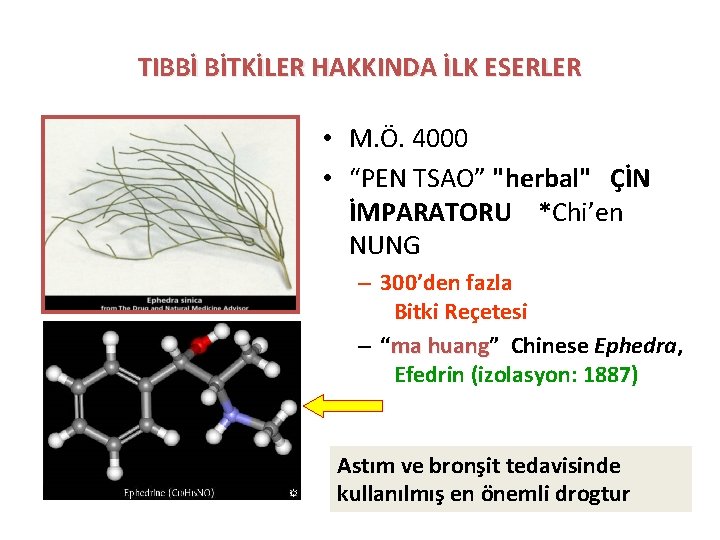
TIBBİ BİTKİLER HAKKINDA İLK ESERLER • M. Ö. 4000 • “PEN TSAO” "herbal" ÇİN İMPARATORU *Chi’en NUNG – 300’den fazla Bitki Reçetesi – “ma huang” huang Chinese Ephedra, Efedrin (izolasyon: 1887) Astım ve bronşit tedavisinde kullanılmış en önemli drogtur

Ephedra Ephedrine • Ephedra E. sinica, known in Chinese as ma huang has been used in traditional Chinese medicine for 5, 000 years for the treatment of asthma and hay fever, as well as for the common cold.

Ephedra Ephedrine • Several additional species belonging to the genus Ephedra have traditionally been used for a variety of medicinal purposes, and are a possible candidate for the Soma plant of Indo Iranian religion. • Native Americans and Mormon pioneers drank a tea brewed from an Ephedra, called Mormon Tea. Ephedra nevadensis Soma (Sanskrit sóma), or Haoma (Avestan), from Proto Indo Iranian *sauma-, was a ritual drink of importance among the early Indo Iranians, and the subsequent Vedic and greater Persian cultures.

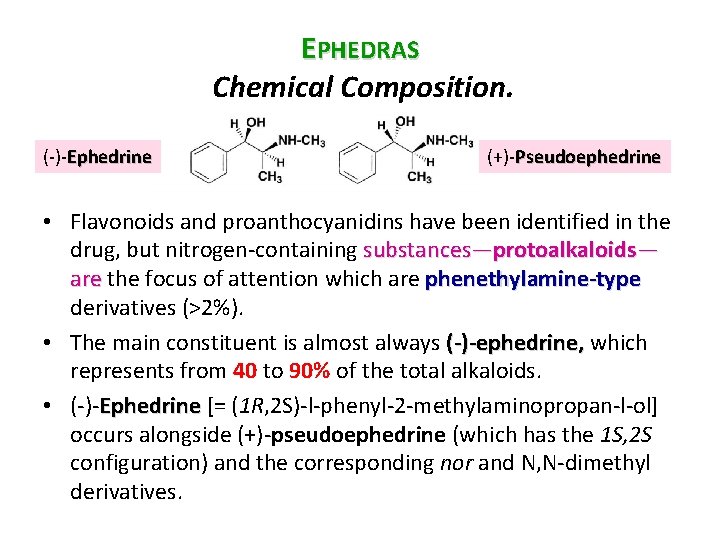
EPHEDRAS Chemical Composition. ( ) Ephedrine (+) Pseudoephedrine • Flavonoids and proanthocyanidins have been identified in the drug, but nitrogen containing substances—protoalkaloids— are the focus of attention which are phenethylamine-type derivatives (>2%). • The main constituent is almost always (-)-ephedrine, which represents from 40 to 90% of the total alkaloids. • ( ) Ephedrine [= (1 R, 2 S) l phenyl 2 methylaminopropan l ol] occurs alongside (+) pseudoephedrine (which has the 1 S, 2 S configuration) and the corresponding nor and N, N dimethyl derivatives.

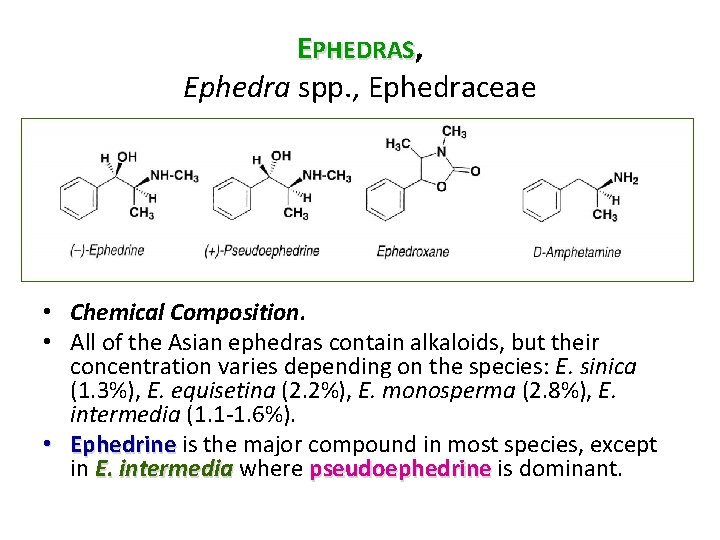
EPHEDRAS, Ephedra spp. , Ephedraceae • Chemical Composition. • All of the Asian ephedras contain alkaloids, but their concentration varies depending on the species: E. sinica (1. 3%), E. equisetina (2. 2%), E. monosperma (2. 8%), E. intermedia (1. 1 1. 6%). • Ephedrine is the major compound in most species, except in E. intermedia where pseudoephedrine is dominant.

EPHEDRAS, Ephedra spp. , Ephedraceae Ephedroxane • The drug also contains traces of cyclic compounds: 5 phenyloxazolidines and ephedroxane (a 3, 4 dimethyl 5 phenyloxazolidone). • The stems also contain a small amount of an alkaloid derived from spermidine, namely orantine, whose structure is very closely related spermidine to that of the macrocycles described in the subterranean parts of some species in the genus. • The roots of several species contain an imidazole derivative (feruloyl histamine), alkaloidal macrocycles derived from spermine (ephedradines A D), and dimeric flavonoids: bisflavanols (mahuannines) and flavano flavonols.

Ephedrine Pharmacological Activity • Pharmacological Activity. • Ephedrine is an indirect sympathomimetic. • Structurally very close to adrenaline, it triggers the release of endogenous catecholamines from the post ganglionic sympathetic fibers. • Ephedrine – stimulates cardiac automaticity and has a positive automaticity inotropic activity; – accelerates respiration and increases its intensity; – is a bronchodilator and a stimulant of the brain stem respiration center; – decreases the contractility of the bladder.

Ephedrine Pharmacological Activity • Ephedrine – is not metabolized much, can be used orally, and its duration of action is longer than that of adrenaline. – is well resorbed and highly lipophilic; – crosses the blood brain barrier and, by releasing mediators centrally, has a stimulating psychic effect: stimulation of the attention and ability to concentrate, – decrease in the sensation of fatigue and the need for sleep. • High doses can cause headaches, anxiety, tremors, insomnia, and psychotic manifestations; redness of the face; nausea; tachycardia and precordial pain; sweating; urinary retention, and more • Ephedroxane and (+)-pseudoephedrine are experimental anti-inflammatory agents

USES OF EPHEDRAS, Ephedra spp. , Ephedraceae • In France, its uses are very limited. • In Germany, E. sinica can be used by the oral route, but only for a short time. • In Asia, the drug has been used for about 5 millenia. Mahuang consists of the stems of E. sinica, E. intermedia, and E. equisetina, and is official in the People's Republic of China where it is used as an antiasthmatic, sudorific. antiasthmatic diuretic, and diuretic sudorific • The Chinese Pharmacopoeia also describes mahuanggen (ephedra root), a drug reputed to be an antisudorific and used as such. • In the United States, ephedras and ephedrine have been presented for a few years as potential aids in weight loss, a claim based on a loss hypothetical stimulating action on the combustion of fats.

USES OF EPHEDRAS, Ephedra spp. , Ephedraceae • Ephedrine can be converted chemically into methcathinone and metamphetamine, two illicit ( illegal) substances. metamphetamine illegal • Naturally, this has led several states in the U. S. to enact restrictive legislation. • The proliferation of products based on mahuang and/or ephedrine has caused an increase in the number of case reports of more or less serious side effects and in 1997, the FDA proposed detailed labeling requirements for ephedra based dietary supplements: warnings against prolonged used and against combinations with products such as caffeine, limited claims, information on side effects, and so forth. • The directions for using these dietary supplements must not lead the consumer to take more than 8 mg per unit dose and 24 mg/24 hours.

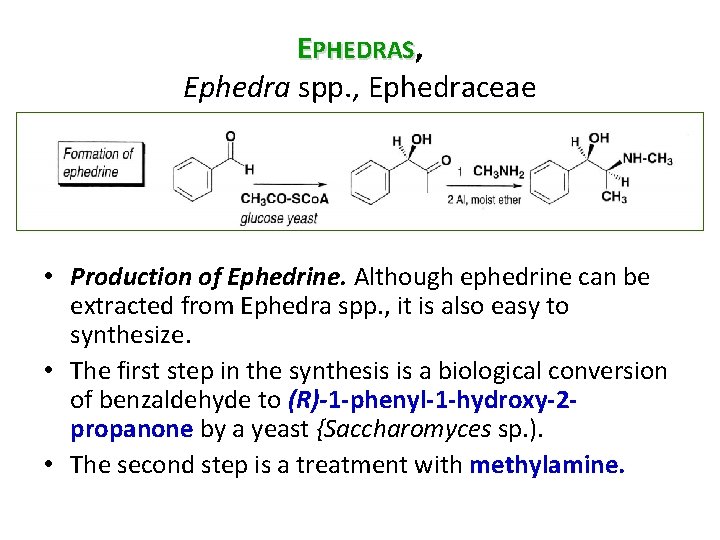
EPHEDRAS, Ephedra spp. , Ephedraceae • Production of Ephedrine. Although ephedrine can be extracted from Ephedra spp. , it is also easy to synthesize. • The first step in the synthesis is a biological conversion of benzaldehyde to (R)-1 -phenyl-1 -hydroxy-2 propanone by a yeast {Saccharomyces sp. ). • The second step is a treatment with methylamine.

EPHEDRAS: EPHEDRINE Uses of Ephedrine • Ephedrine hydrochloride has long been used to treat the acute attack of asthma • Its multiple activities, – numerous contraindications (coronary insufficiency, arterial hypertension, closed angle glaucoma, hyperthyroidism), closed angle glaucoma, hyperthyroidism – drug interactions (MAO inhibitors, tricyclic antidepressants), MAO inhibitors, tricyclic antidepressants – the required precautions (prostatic hypertrophy, cardiac insufficiency, diabetes), diabetes – potential adverse effects (tachycardia, headaches, sweating, agitation, insomnia, anxiety), insomnia, anxiety – as well as the fact that the effects wear out if the doses are repeated in close time proximity (tachyphylaxis) tachyphylaxis • have led to the virtual (almost) abandon of this compound as a bronchodilator and analeptic • However, it remains available in some European countries like France for this indication, particularly in combinations (with theophylline, caffeine, and others).

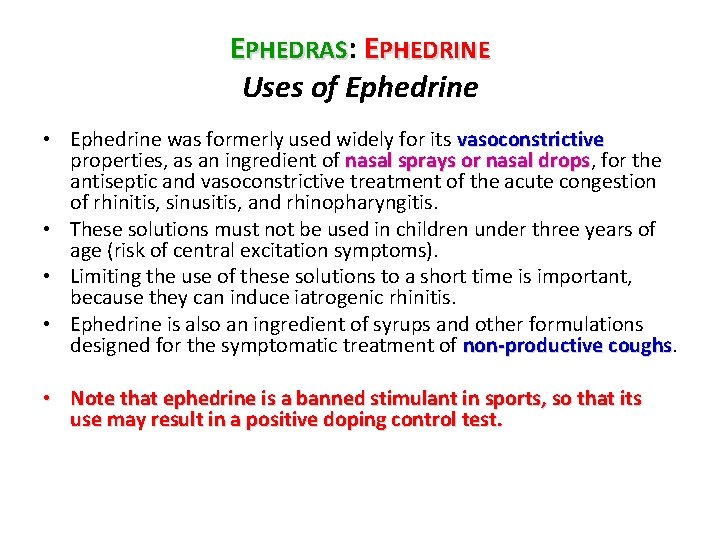
EPHEDRAS: EPHEDRINE Uses of Ephedrine • Ephedrine was formerly used widely for its vasoconstrictive properties, as an ingredient of nasal sprays or nasal drops, for the drops antiseptic and vasoconstrictive treatment of the acute congestion of rhinitis, sinusitis, and rhinopharyngitis. • These solutions must not be used in children under three years of age (risk of central excitation symptoms). • Limiting the use of these solutions to a short time is important, because they can induce iatrogenic rhinitis. • Ephedrine is also an ingredient of syrups and other formulations designed for the symptomatic treatment of non-productive coughs • Note that ephedrine is a banned stimulant in sports, so that its use may result in a positive doping control test.

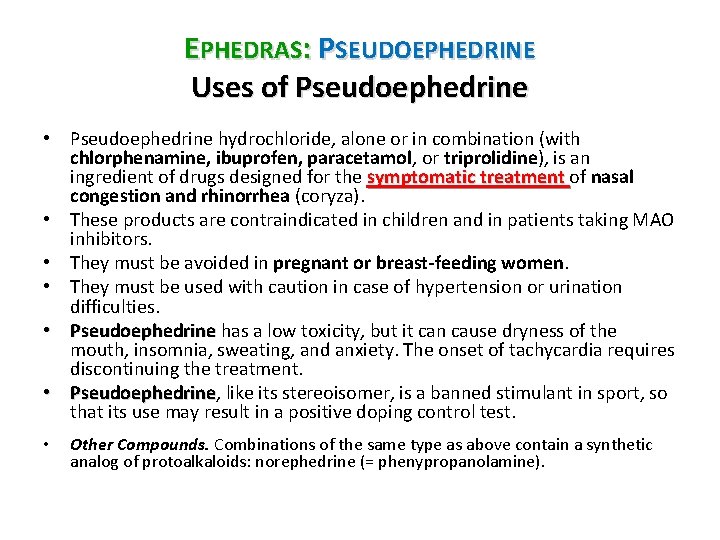
EPHEDRAS: PSEUDOEPHEDRINE Uses of Pseudoephedrine • Pseudoephedrine hydrochloride, alone or in combination (with chlorphenamine, ibuprofen, paracetamol, or triprolidine), is an ingredient of drugs designed for the symptomatic treatment of nasal congestion and rhinorrhea (coryza). • These products are contraindicated in children and in patients taking MAO inhibitors. • They must be avoided in pregnant or breast-feeding women. • They must be used with caution in case of hypertension or urination difficulties. • Pseudoephedrine has a low toxicity, but it can cause dryness of the mouth, insomnia, sweating, and anxiety. The onset of tachycardia requires discontinuing the treatment. • Pseudoephedrine, like its stereoisomer, is a banned stimulant in sport, so Pseudoephedrine that its use may result in a positive doping control test. • Other Compounds. Combinations of the same type as above contain a synthetic analog of protoalkaloids: norephedrine (= phenypropanolamine).

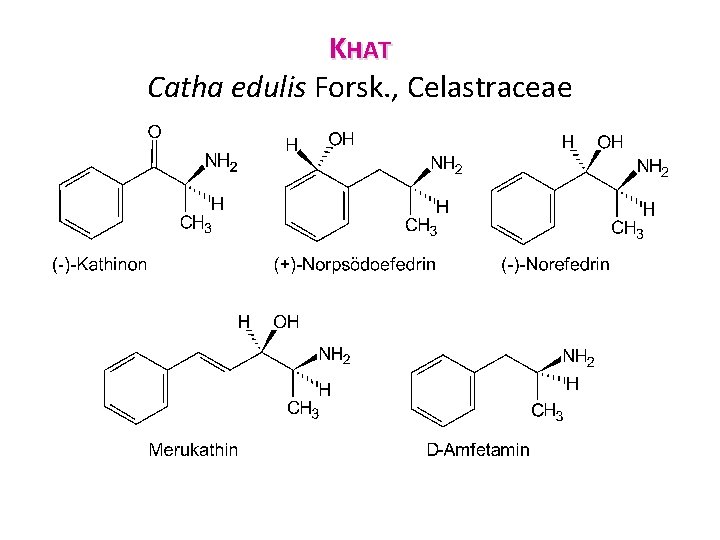
KHAT Catha edulis, Celastraceae • Khat (or cath, chat, jat, tschatt, and so forth) is a shrub of modest size in arid areas (1 2 m), but it can reach 10 m in the tropics. • The leaves are highly polymorphic and indeciduous. Also known as Abyssinian tea, it is native to the horn of Africa (but some think that it originated in Yemen).

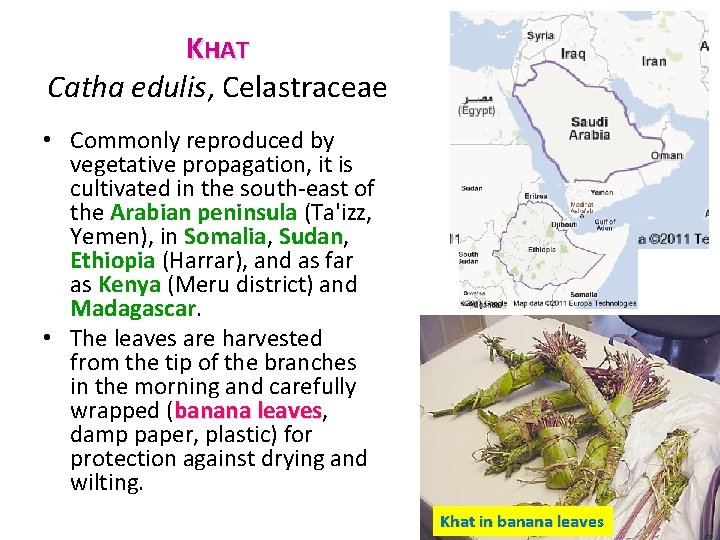
KHAT Catha edulis, Celastraceae • Commonly reproduced by vegetative propagation, it is cultivated in the south east of the Arabian peninsula (Ta'izz, Yemen), in Somalia, Sudan, Ethiopia (Harrar), and as far as Kenya (Meru district) and Madagascar. • The leaves are harvested from the tip of the branches in the morning and carefully wrapped (banana leaves, leaves damp paper, plastic) for protection against drying and wilting. Khat in banana leaves

KHAT Catha edulis Forsk. , Celastraceae • Chemical Composition. Chemically, the leaf contains flavonoids, some essential oil, complex polyesters of polyhydroxylated dihydroagarofurans (cathedulines), and arylalkylamines (the khatamines), which are responsible khatamines for the activity of the drug. • In the fresh and young leaves, the chief constituent is cathinone, in other words (S)-α-aminopropiophenone. In -aminopropiophenone the dried drag and in older leaves, this (-)-cathinone has been converted to an 80 20 mixture of (S, S)-(+)norpseudoephedrine and (R, S)-(-)-norephedrine. )-(-)-norephedrine

KHAT Catha edulis Forsk. , Celastraceae

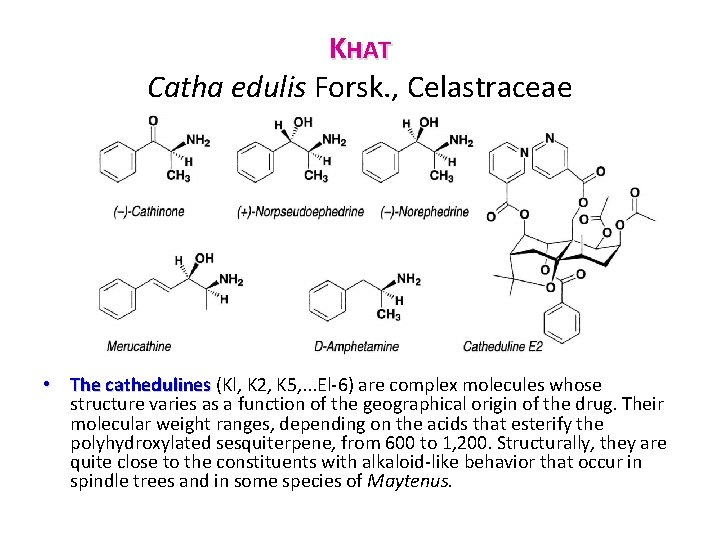
KHAT Catha edulis Forsk. , Celastraceae • Chemical Composition. Chemically, the leaf contains flavonoids, some essential oil, complex polyesters of polyhydroxylated dihydroagarofurans (cathedulines), and arylalkylamines (the khatamines), which are responsible for the activity of the drug. In the fresh and young leaves, the chief constituent is cathinone, in other words (S) α aminopropiophenone. In the dried drag and in older leaves, this ( ) cathinone has been converted to an 80 20 mixture of (S, S) (+) norpseudoephedrine and (R, S) ( ) norephedrine. • Fresh drug from northern Kenya also contains the C 6 -C 4 homologs of these phenylpropylamines: (R, S) (+) merucathine, (S) (+) merucathinone, and (S, S) ( ) pseudomeracathine. • The phenylpropylamine content is maximal in the young shoots and appears to depend on the geographical origin: the cathinone level is reported to range from 9 to 330 mg/100 g depending on the harvest location. A range of variation just as wide has been observed for norephedrine and norpseudoephedrine.

KHAT, CATHA Catha edulis Forsk. , Celastraceae Drog ayrıca C 6 C 4 homologları olan merukatin, merukatinon ve merukatinon pseudomerukatin gibi bileşikleri de taşır. pseudomerukatin

KHAT Catha edulis Forsk. , Celastraceae • The cathedulines (Kl, K 2, K 5, . . . El 6) are complex molecules whose structure varies as a function of the geographical origin of the drug. Their molecular weight ranges, depending on the acids that esterify the polyhydroxylated sesquiterpene, from 600 to 1, 200. Structurally, they are quite close to the constituents with alkaloid like behavior that occur in spindle trees and in some species of Maytenus.

KHAT Catha edulis Forsk. , Celastraceae • Pharmacological Activity - Toxicity. Pharmacologically, the activity of ( ) cathinone is qualitatively quite comparable to that of D amphetamine: it causes anorexia, hyperthermia, respiratory stimulation, mydriasis, arrhythmia, and hypertension. • This amine induces the release of catecholamines from storage. Its effects on the central nervous system depend in part on the subject's environment; they are characterized by a subjective and euphoric sensation of increased energy, well being, self confidence, mental acuity, and ease in thought formation. Objectively, slight euphoria can be observed in a talkative and sometimes hyperactive subject. Later on, undesired effects can appear: insomnia, nervousness, and nightmares. • In very rare cases, khat can induce a toxic psychosis, probably by potentiating a prepsychotic condition. Depression is then observed, and even schizophreniform or paranoid symptoms.

KHAT Catha edulis Forsk. , Celastraceae • Use of Khat. The fresh leaves, sold within 24 hours of harvest, constitute a masticatory known for its stimulating properties. In some countries (Yemen), khat use is an ancient custom and it is practiced at social events, thereby strengthening social bonds. In other countries, khat is used mainly to seek the pharmacological effects of the alkaloids (to suppress the appetite and to combat fatigue). • Traditionally, the leaves (50 200 g) are chewed one by one, kept in the mouth for a while, then most often spit out.

KHAT Catha edulis Forsk. , Celastraceae • In the early 1990 s, Brenneisen and El Sohly estimated the number of daily users of khat leaves to be between two and eight million (northeast Africa, Yemen). • Khat use is officially forbidden in some countries (Saudi Arabia, Sudan, Somalia) and more or less tolerated in other countries. Tolerated in Yemen where the authorities ban alcohol, khat is thought to be consumed daily by 50% of adult men. • Cultivated without restriction in Ethiopia, it makes a massive contribution to the national economy: it is widely consumed there, and it is also exported to Djibouti where 90% of men and 10% of women are thought to be regular or occasional users.

Isoquinoline Alkaloids Simple Tetrahydroisoquinolines • PEYOTE • Lophophora williamsii – Cactaceae • Considered a divine plant by the Aztecs, this cactus is a particularly potent hallucinogen. This is the "plant that makes the eyes amazed", in other words causes visual hallucinations, due to the CNS activity of a phenethylamine alkaloid, mescaline.

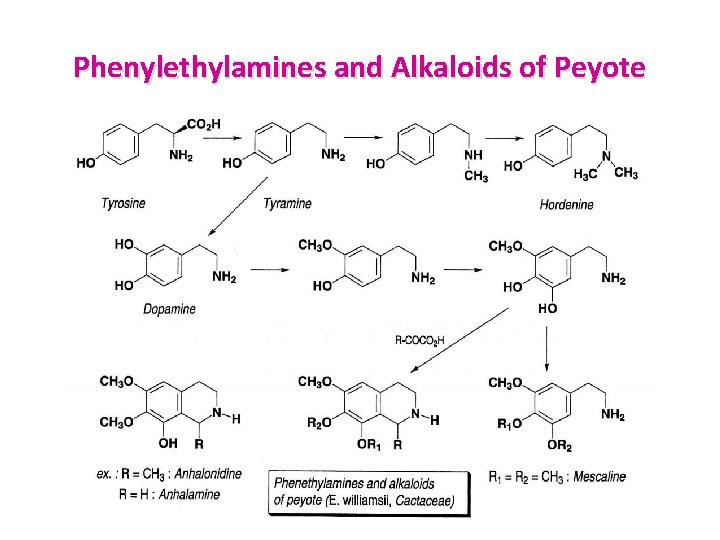
Simple Tetrahydroisoquinolines PEYOTE: Lophophora williamsii – Cactaceae • The drug contains a large amount of mucilage and about fifty nitrogen containing compounds: phenethylamines and tetrahydroisoquinolines (fresh plant: 0. 5 1%, mescal buttons: 6%). • The phenethylamines include mescaline (= 3, 4, 5 tri methoxy phenethylamine) and its derivatives (N formyl, N acetyl, N methyl), hordenine, 3, 4 hordenine 3 -demethyl- and 3 -demethylmescaline, tyramine and its derivatives methylated on the nitrogen atom, and dopamine • The tetrahydroisoquinoline alkaloids are anhalamine, anhalonidine, anhalidine, pellotine, and lophophorine. anhalonidine, anhalidine, pellotine, lophophorine • They arise from the condensation of a phenethylamine with an a ketoacid (glyoxylic acid, pyruvic acid).

Phenylethylamines and Alkaloids of Peyote

Simple Tetrahydroisoquinolines PEYOTE: Lophophora williamsii – Cactaceae • The ingestion of peyote essentially causes psychic effects. • Mescaline has clinical effects resembling those of LSD (lysergic acid diethylamide): psychic, cognitive, and physical. – Note in particular a distortion of the perception of shapes, an intensification of colors, auditory hallucinations, and a slowing in the perception of time; the intensity and the nature of the effects are highly dependent on the environment and the intellect of the subject (for example, his or her artistic sensitivity). • The physical symptoms that accompany these hallucinations are mydriasis, tachycardia, bradypnea, a sensation of change in temperature, nausea, and possibly agitation and anxiety. At high doses, memory loss, hypertensive encephalo pathy, and intracranial hemorrhage may be observed.

Isoquinoline Alkaloids Benzyltetrahydroisoquinolines • I. Simple Benzylisoquinolines – Papaverine • II. Bisbenzyltetrahydroisoquinolines – Curare – Other Naturally occurring Substances with Curare like Activity • III. Aporphinoids • IV. Protoberberines and Derivatives • V. Morphinan Alkaloids – Introduction: Biosynthetic Origin – Opium Poppy: Opium • Chemical Composition – – – Opium Morphine Codeine Other Alkaloids Semisynthetic Alkaloids

Isoquinoline Alkaloids Benzyltetrahydroisoquinolines • Introduction: The alkaloids derived from 1 benzylisoquinoline are surpassed, in structural benzylisoquinoline diversity • Oxidative Coupling • The above structural diversity results from the diversity broad reactivity of phenolics, particularly their coupling reactions via radical intermediates: this is the classic oxidative coupling of phenols.

Isoquinoline Alkaloids Benzyltetrahydroisoquinolines Example of Oxidative coupling Formation of the phenoxy radical, mesomerization, • The phenoxy radical, formed upon oxidation of the phenate radical ion, and stabilized by resonance, is highly reactive: reactive depending on whether the coupling involves the phenoxy radical and its mesomers, or only the latter, the result is either the formation of – a biphenylether bond (Ar-O-Ar), or a – biphenyl carbon-carbon bond (HO-Ar-Ar-OH).

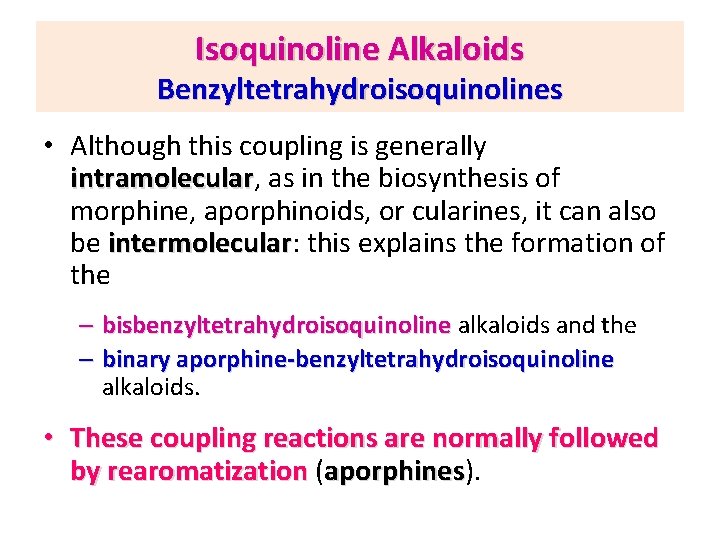
Isoquinoline Alkaloids Benzyltetrahydroisoquinolines • Although this coupling is generally intramolecular, as in the biosynthesis of intramolecular morphine, aporphinoids, or cularines, it can also be intermolecular: this explains the formation of intermolecular the – bisbenzyltetrahydroisoquinoline alkaloids and the – binary aporphine-benzyltetrahydroisoquinoline alkaloids. • These coupling reactions are normally followed by rearomatization (aporphines). aporphines

Isoquinoline Alkaloids Benzyltetrahydroisoquinolines Alkyl substitution at the coupling site leads to a rearrangment, after which rearomatization is possible • In a few cases, however, there is no simple pathway for rearomatization, and rearrangements take place instead, which can lead to new, more or less profound structural variations (morphinan and erythrinane alkaloids).

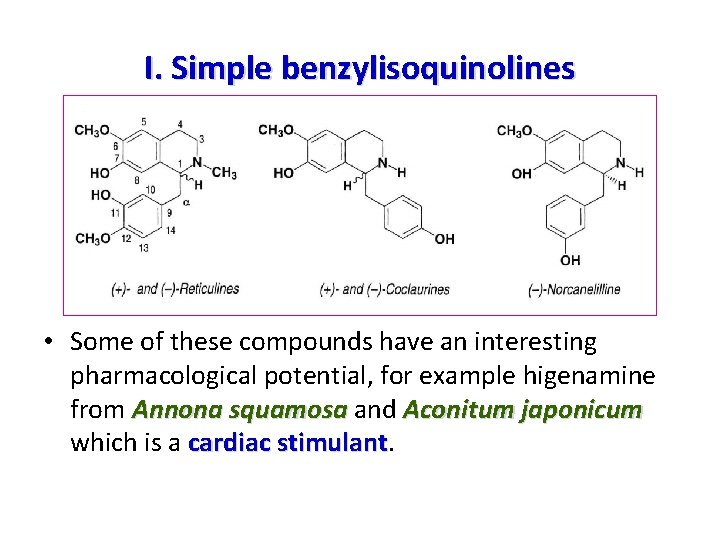
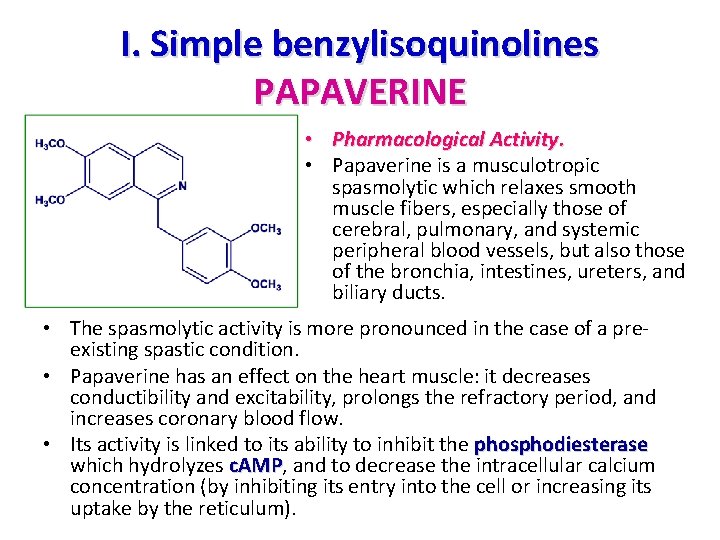
I. Simple benzylisoquinolines • The quasi totality of these simple compounds are 1, 2, 3, 4 tetrahydro derivatives, in other words 1, 2, 3, 4 tetrahydro derivatives benzyltetrahydroisoquinolines • In a few exceptional cases, they are aromatic: one example is papaverine • All of these compounds have, for biogenetic reasons, a 6, 7 -disubstituted isoquinoline nucleus and a mono-, di -, or trisubstituted benzyl moiety: the most common derivatives are of the coclaurine type (12 -monosubstituted) and reticuline type ( 11, 12 -disubstituted). substituted reticuline 11, 12 -disubstituted • They are the precursors of all other isoquinoline alkaloids.

I. Simple benzylisoquinolines • Some of these compounds have an interesting pharmacological potential, for example higenamine from Annona squamosa and Aconitum japonicum which is a cardiac stimulant

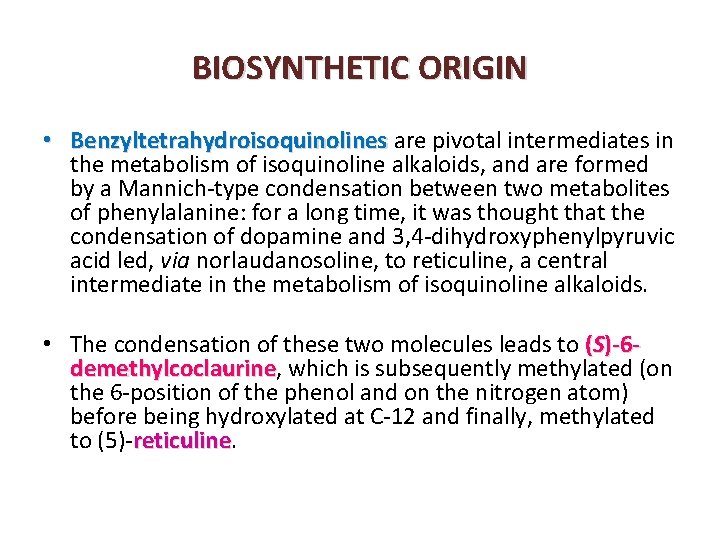
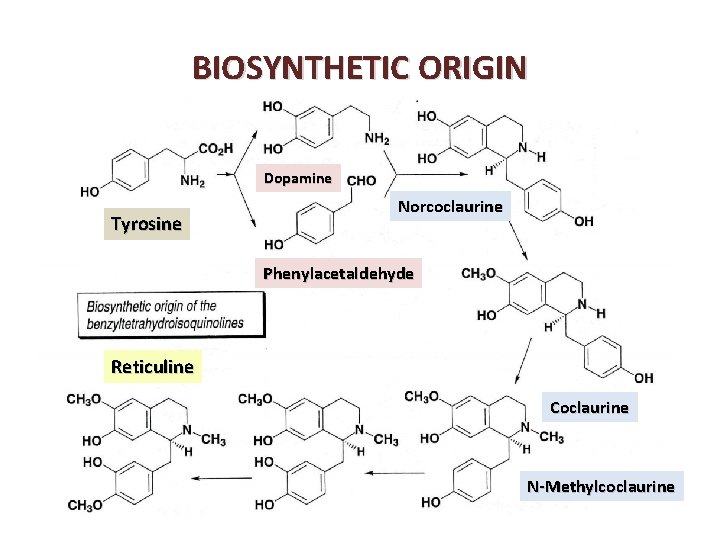
BIOSYNTHETIC ORIGIN • Benzyltetrahydroisoquinolines are pivotal intermediates in Benzyltetrahydroisoquinolines the metabolism of isoquinoline alkaloids, and are formed by a Mannich type condensation between two metabolites of phenylalanine: for a long time, it was thought that the condensation of dopamine and 3, 4 dihydroxyphenylpyruvic acid led, via norlaudanosoline, to reticuline, a central intermediate in the metabolism of isoquinoline alkaloids. • The condensation of these two molecules leads to (S)-6 demethylcoclaurine, which is subsequently methylated (on demethylcoclaurine the 6 position of the phenol and on the nitrogen atom) before being hydroxylated at C 12 and finally, methylated to (5) reticuline

BIOSYNTHETIC ORIGIN Dopamine Tyrosine Norcoclaurine Phenylacetaldehyde Reticuline Coclaurine N-Methylcoclaurine

I. Simple benzylisoquinolines PAPAVERINE • Pharmacological Activity. • Papaverine is a musculotropic spasmolytic which relaxes smooth muscle fibers, especially those of cerebral, pulmonary, and systemic peripheral blood vessels, but also those of the bronchia, intestines, ureters, and biliary ducts. • The spasmolytic activity is more pronounced in the case of a pre existing spastic condition. • Papaverine has an effect on the heart muscle: it decreases conductibility and excitability, prolongs the refractory period, and increases coronary blood flow. • Its activity is linked to its ability to inhibit the phosphodiesterase which hydrolyzes c. AMP, and to decrease the intracellular calcium c. AMP concentration (by inhibiting its entry into the cell or increasing its uptake by the reticulum).

I. Simple benzylisoquinolines PAPAVERINE: Uses • Uses. It is still fairly widely used as"vasodilator and antiischemic" in the curative or preventive treatment of ischemic cerebral circulatory insufficiency. • In addition to being indicated as a smooth muscle relaxant (injectable solution at 4%) and for the symptomatic treatment of the intermittent claudication due to chronic occlusive arterial disease of the lower limbs, it is proposed: – 1. to improve certain symptoms of senility (e. g. , loss of attention and memory); – 2. for the symptoms of ischemia in the eye. It is also used for vertigo in the elderly and to treat the sequelae of cerebrovascular accidents.

I. Simple benzylisoquinolines PAPAVERINE: Uses • Uses. • Contraindications include intra cranial hypertension, parkinsonism, and intracardiac conductibility alterations, but papaverine is not a hypotensive agent and only rarely has side effects (potential tachycardia, constipation, altered transaminases, phosphatases, and bilirubinemia). • For the same indications as above, papaverine is sometimes combined with other compounds (e. g. , butalamine). This alkaloid is also an ingredient of combinations designed to treat capillary fragility (e. g. , combinations with hesperidin methyl chalcone, ascorbic acid, and ethoxazorutin). • As an antispasmodic, it is a component of proprietary drugs designed to relieve the symptoms of functional colopathy, particularly flatulence and diarrhea.
 Lophophora
Lophophora Alkaloids derived from tyrosine
Alkaloids derived from tyrosine Hsan
Hsan Phenylalanine metabolism
Phenylalanine metabolism Neutral amino acids
Neutral amino acids Modular architecture
Modular architecture Pyruvate amino acid synthesis
Pyruvate amino acid synthesis Tyrosine letter code
Tyrosine letter code Receptor tyrosine kinase structure
Receptor tyrosine kinase structure Tyrosine kinase pathway
Tyrosine kinase pathway Pyridine piperidine alkaloids
Pyridine piperidine alkaloids Aprophine
Aprophine Norlupinane
Norlupinane Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Ergot alkaloids
Ergot alkaloids Biological importance of alkaloids
Biological importance of alkaloids Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Protoalkaloid
Protoalkaloid Purine alkaloids
Purine alkaloids Pyrimidine examples
Pyrimidine examples Capsicum
Capsicum Tropane alkaloids
Tropane alkaloids Ergot alkaloids alpha blockers
Ergot alkaloids alpha blockers Tocolytic drugs
Tocolytic drugs Base and derived quantities
Base and derived quantities Fundamental starting position
Fundamental starting position Physical quantities units and measurement
Physical quantities units and measurement Basic engineering and science
Basic engineering and science Difference between fundamental and derived unit
Difference between fundamental and derived unit Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Starting and derived positions
Starting and derived positions Acuity charting forms
Acuity charting forms Mosby items and derived items
Mosby items and derived items Mosby
Mosby Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Autonomic drugs
Autonomic drugs Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Absorption atelectasis
Absorption atelectasis Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Derived data types in c
Derived data types in c Pic microcontroller and embedded systems by mazidi
Pic microcontroller and embedded systems by mazidi The term audit originated from the latin word audit
The term audit originated from the latin word audit Suffixes of noun
Suffixes of noun Si derived unit of pressure scientist
Si derived unit of pressure scientist Satellite derived winds
Satellite derived winds