PHARMACOGNOSY III th 6 d e n r






















- Slides: 38

PHARMACOGNOSY III th 6 d e n r th a u 5 lect

5. Isoquinoline Alkaloids 6 1. Papaver somniferum (Papaveraceae) Papaverine, narceine, narcotine, Morphine, thebaine and codeine. Morphine is named after Morpheus ( the god of sleep). These potent alkaloids are obtained from the milky latex sap of the mature seed capsule of the opium poppy. The raw opium sap drips from fresh cuts into the pericarp ﻏﻼﻑ ﺍﻟﺜﻤﺮﺓ of the capsules, and upon oxidation and solidification in the air it turns black.

• Chief alkaloids morphine (3 -23%), narcotine (2 -10%), codeine (0. 2 -3. 5%), papaverine (0. 5 -3%), thebaine (0. 2 -1%). Morphine is acetylated to produce diacetylmorphine(heroin). • During the late 1800 s and early 1900 s, the narcotic alkaloids morphine and codeine were available in over-the-counter drugs, such as cough syrups and teething medicines. In fact, there are cases of young children becoming addicted to opiates.

• Opium is obtained by slightly incising the seed capsules of the poppy after the plant’s flower petals have fallen. The slit seedpods exude a milky latex that coagulates and changes colour, turning into a gumlike brown mass upon exposure to air. • This raw opium may be ground into a powder, sold as lumps, cakes, or bricks, or treated further to obtain derivatives such as morphine, codeine, and heroin. • Opium and the drugs obtained from it are called (opiates).

• Mature seed pod of the opium poppy (Papaver somniferum) with milky latex sap dripping from a recent cut. The latex sap contains a mixture of naturally-occurring narcotic alkaloids. • The four economically significant alkaloids of opium are morphine, codeine, thebaine and papaverine. • Opium alkaloids are of two types, depending on chemical structure and action. Morphine, codeine, and thebaine, which represent one type, act upon the central nervous system and are analgesic, narcotic, and potentially addicting compounds.

Papaverine, noscapine (formerly called narcotine), and most of the other opium alkaloids act only to relax involuntary (smooth) muscles. • Opioid receptors are distributed widely in brain and found in spinal cord and peripheral sensory and autonomic nerves. Endogenous opioid systems have a functional role in modulating pain perception; opioid agonists are therefore potent analgesics. • Opioid receptors are also present in hypothalamus, where they influence temperature regulation and control of hormonal secretion. •

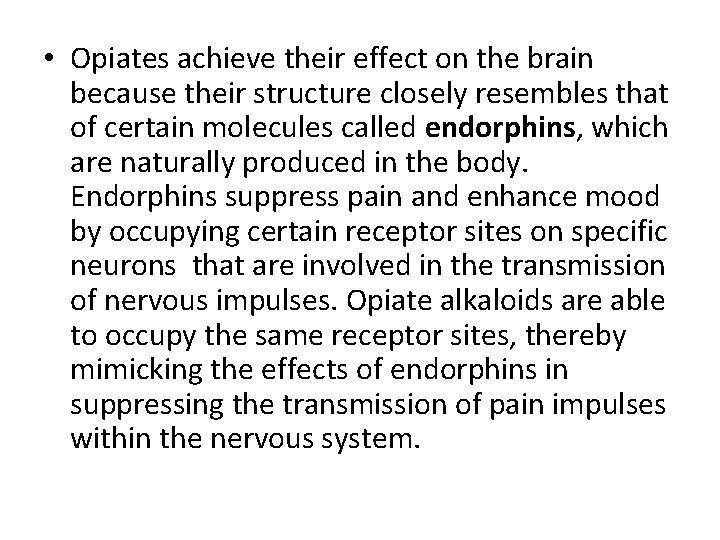
• Opiates achieve their effect on the brain because their structure closely resembles that of certain molecules called endorphins, which are naturally produced in the body. Endorphins suppress pain and enhance mood by occupying certain receptor sites on specific neurons that are involved in the transmission of nervous impulses. Opiate alkaloids are able to occupy the same receptor sites, thereby mimicking the effects of endorphins in suppressing the transmission of pain impulses within the nervous system.

Physiological Actions Of Opiates • Opiates (e. g. , morphine, codeine, and thebaine) exert their main effects on the brain and spinal cord. Their principal action is to relieve or suppress pain. The drugs also alleviate anxiety; induce relaxation, drowsiness, and sedation; and may impart a state of euphoria or other enhanced mood. Opiates also have important physiological effects: they slow respiration and heartbeat, suppress the cough reflex, and relax the smooth muscles of the gastrointestinal tract.

• Opiates are addictive drugs; they produce a physical dependence and withdrawal syndroms. • With chronic use, the body develops a tolerance to opiates, so that progressively larger doses are needed to achieve the same effect. • The higher opiates—heroin and morphine—are more addictive than opium or codeine. Opiates are classified as narcotics because they relieve pain, induce sleep, and produce addiction. The habitual use of opium produces physical and mental deterioration and shortens life. An acute overdose of opium causes respiratory depression which can be fatal.

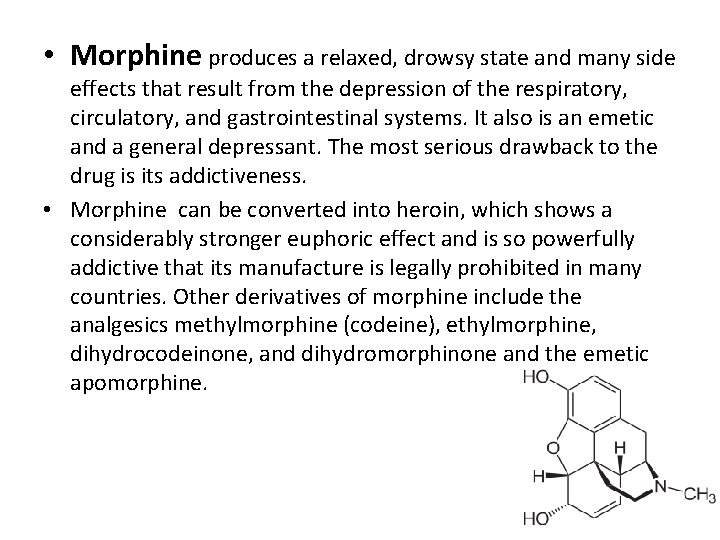
• Morphine produces a relaxed, drowsy state and many side effects that result from the depression of the respiratory, circulatory, and gastrointestinal systems. It also is an emetic and a general depressant. The most serious drawback to the drug is its addictiveness. • Morphine can be converted into heroin, which shows a considerably stronger euphoric effect and is so powerfully addictive that its manufacture is legally prohibited in many countries. Other derivatives of morphine include the analgesics methylmorphine (codeine), ethylmorphine, dihydrocodeinone, and dihydromorphinone and the emetic apomorphine.

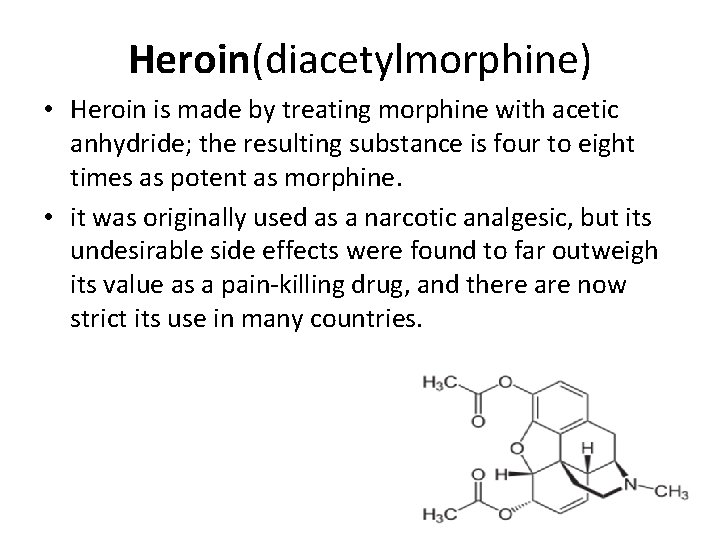
Heroin(diacetylmorphine) • Heroin is made by treating morphine with acetic anhydride; the resulting substance is four to eight times as potent as morphine. • it was originally used as a narcotic analgesic, but its undesirable side effects were found to far outweigh its value as a pain-killing drug, and there are now strict its use in many countries.

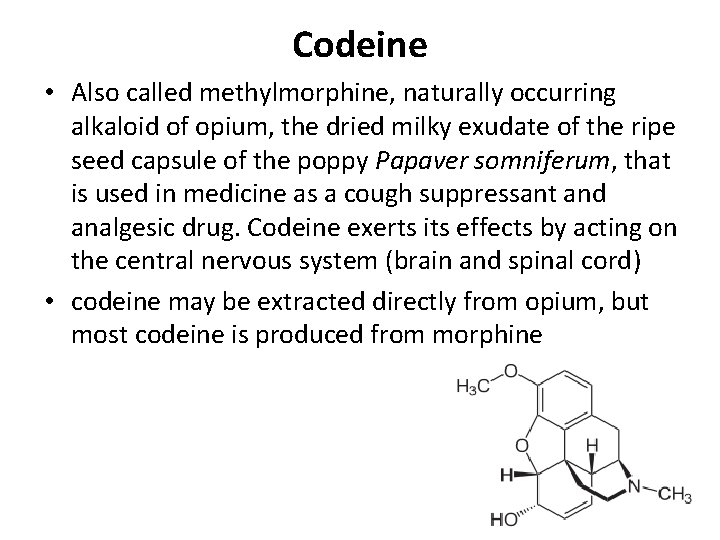
Codeine • Also called methylmorphine, naturally occurring alkaloid of opium, the dried milky exudate of the ripe seed capsule of the poppy Papaver somniferum, that is used in medicine as a cough suppressant and analgesic drug. Codeine exerts its effects by acting on the central nervous system (brain and spinal cord) • codeine may be extracted directly from opium, but most codeine is produced from morphine

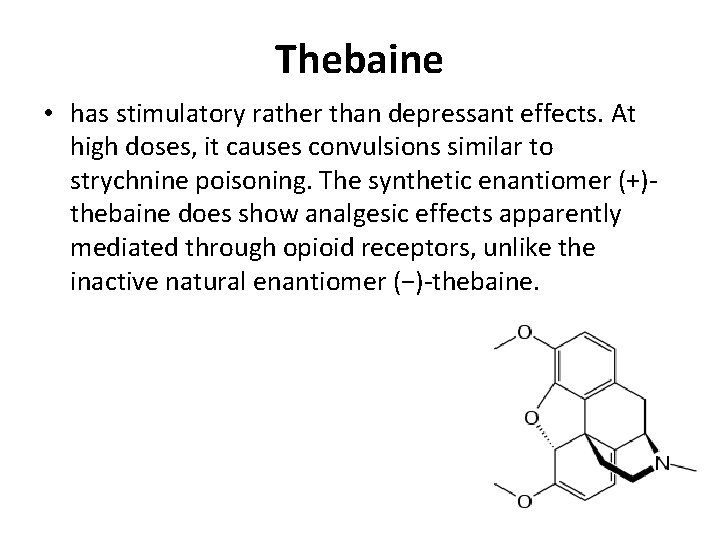
Thebaine • has stimulatory rather than depressant effects. At high doses, it causes convulsions similar to strychnine poisoning. The synthetic enantiomer (+)thebaine does show analgesic effects apparently mediated through opioid receptors, unlike the inactive natural enantiomer (−)-thebaine.

Papaverine Is a benzylisoquinoline alkaloid, and is structurally very different from the morphine, codeine, thebaine group of alkaloids. It has little or no analgesic or a hypnotic property put possesses spasmolytic and vasodilator activity. It has been used in some expectorant preparations, and in the treatment of gastrointestinal spasms.

Noscapine has good antitussive and cough suppressant activity comparable to that of codeine, but no analgesic or narcotic action. Its original name narcotine‘ was changed to reflect this lack of narcotic action. Despite many years of use as a cough suppressant, the finding that noscapine may have teratogenic properties (i. e. may deform a fetus) has resulted in noscapine preparations being deleted. In recent studies, antitumour activity has been noted from noscapine, which binds to tubulin thus arresting cells at mitosis.

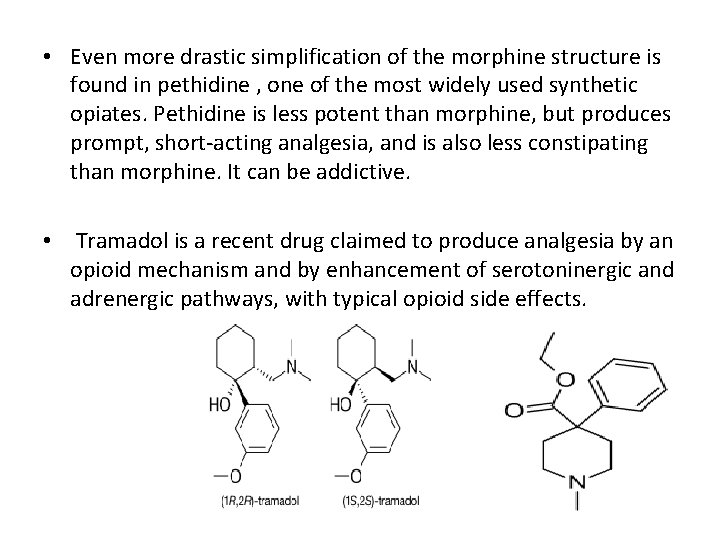
• Even more drastic simplification of the morphine structure is found in pethidine , one of the most widely used synthetic opiates. Pethidine is less potent than morphine, but produces prompt, short-acting analgesia, and is also less constipating than morphine. It can be addictive. • Tramadol is a recent drug claimed to produce analgesia by an opioid mechanism and by enhancement of serotoninergic and adrenergic pathways, with typical opioid side effects.

• 2. Hydrastine, berberine • Numerous genera of the Berberidaceae, Ranunculaceae and Papaveraceae • Hydrastis canadensis(orangeroot)(goldenseal) • They have an antibiotic activity

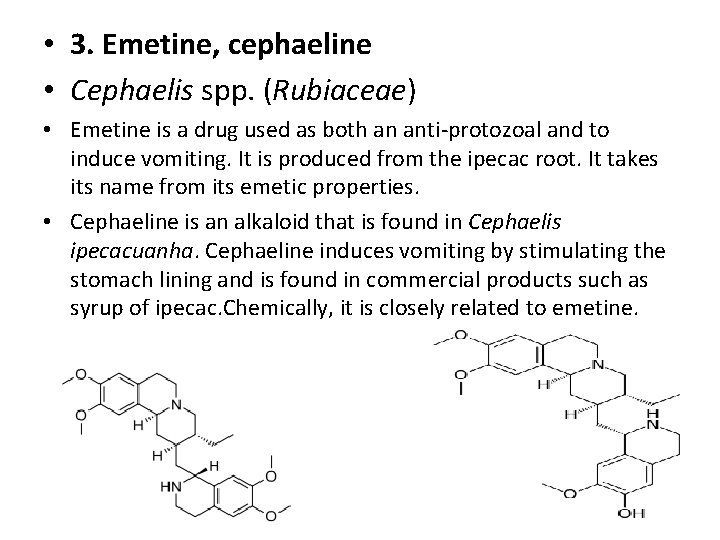
• 3. Emetine, cephaeline • Cephaelis spp. (Rubiaceae) • Emetine is a drug used as both an anti-protozoal and to induce vomiting. It is produced from the ipecac root. It takes its name from its emetic properties. • Cephaeline is an alkaloid that is found in Cephaelis ipecacuanha. Cephaeline induces vomiting by stimulating the stomach lining and is found in commercial products such as syrup of ipecac. Chemically, it is closely related to emetine.

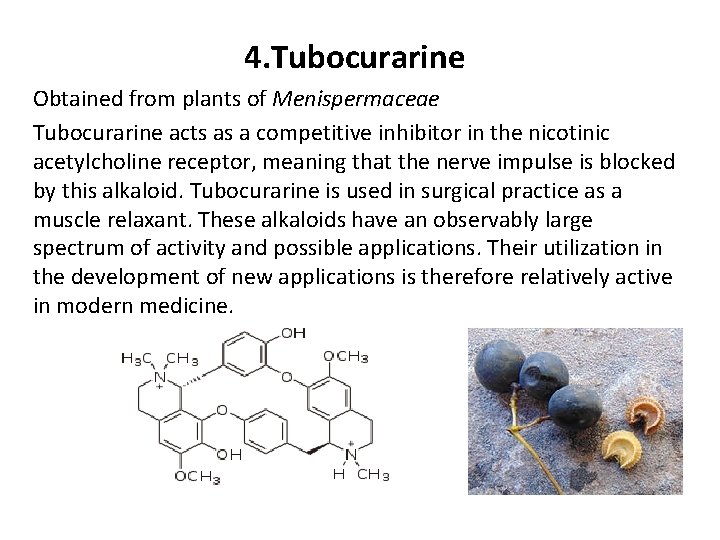
4. Tubocurarine Obtained from plants of Menispermaceae Tubocurarine acts as a competitive inhibitor in the nicotinic acetylcholine receptor, meaning that the nerve impulse is blocked by this alkaloid. Tubocurarine is used in surgical practice as a muscle relaxant. These alkaloids have an observably large spectrum of activity and possible applications. Their utilization in the development of new applications is therefore relatively active in modern medicine.

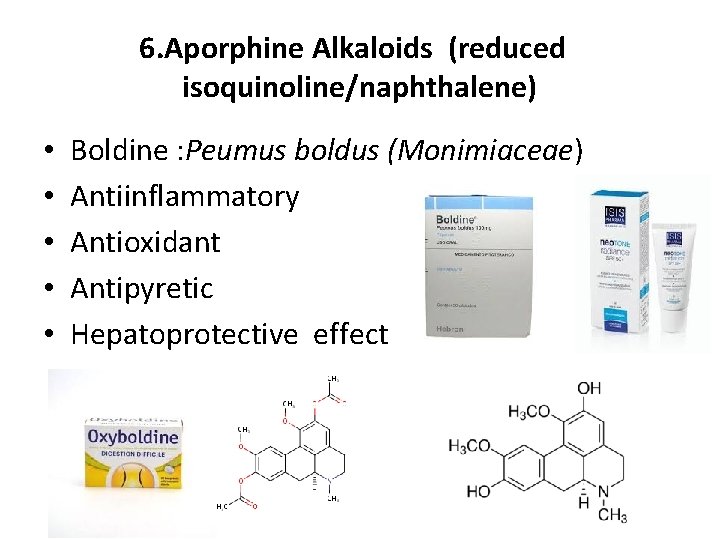
6. Aporphine Alkaloids (reduced isoquinoline/naphthalene) • • • Boldine : Peumus boldus (Monimiaceae) Antiinflammatory Antioxidant Antipyretic Hepatoprotective effect

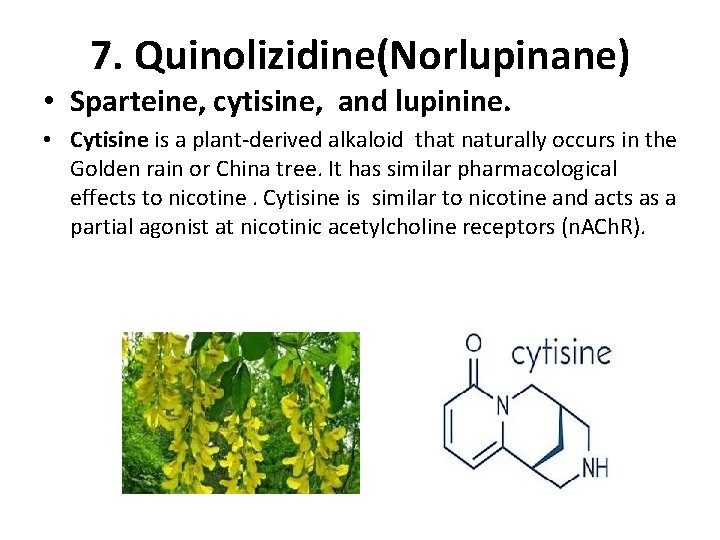
7. Quinolizidine(Norlupinane) • Sparteine, cytisine, and lupinine. • Cytisine is a plant-derived alkaloid that naturally occurs in the Golden rain or China tree. It has similar pharmacological effects to nicotine. Cytisine is similar to nicotine and acts as a partial agonist at nicotinic acetylcholine receptors (n. ACh. R).

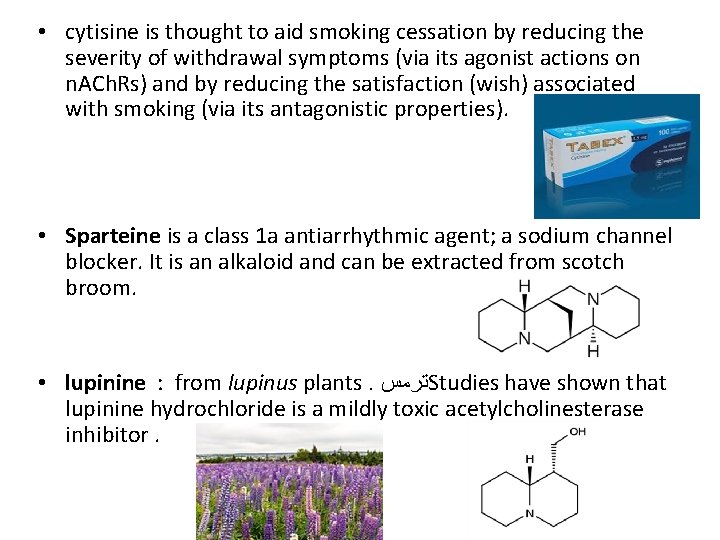
• cytisine is thought to aid smoking cessation by reducing the severity of withdrawal symptoms (via its agonist actions on n. ACh. Rs) and by reducing the satisfaction (wish) associated with smoking (via its antagonistic properties). • Sparteine is a class 1 a antiarrhythmic agent; a sodium channel blocker. It is an alkaloid and can be extracted from scotch broom. • lupinine : from lupinus plants. ﺗﺮﻣﺲ Studies have shown that lupinine hydrochloride is a mildly toxic acetylcholinesterase inhibitor.

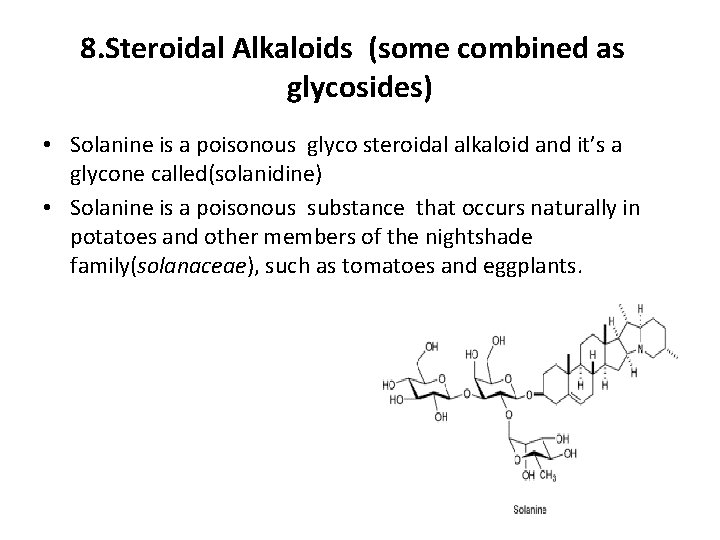
8. Steroidal Alkaloids (some combined as glycosides) • Solanine is a poisonous glyco steroidal alkaloid and it’s a glycone called(solanidine) • Solanine is a poisonous substance that occurs naturally in potatoes and other members of the nightshade family(solanaceae), such as tomatoes and eggplants.

• A very small amount of solanine can be toxic, and in very large doses it can be fatal. In potatoes, the skin will turn green and there will be a very bitter flavor. Symptoms of solanine poisoning include diarrhea and vomiting. • It can be used as precursor for hormonal synthesis.

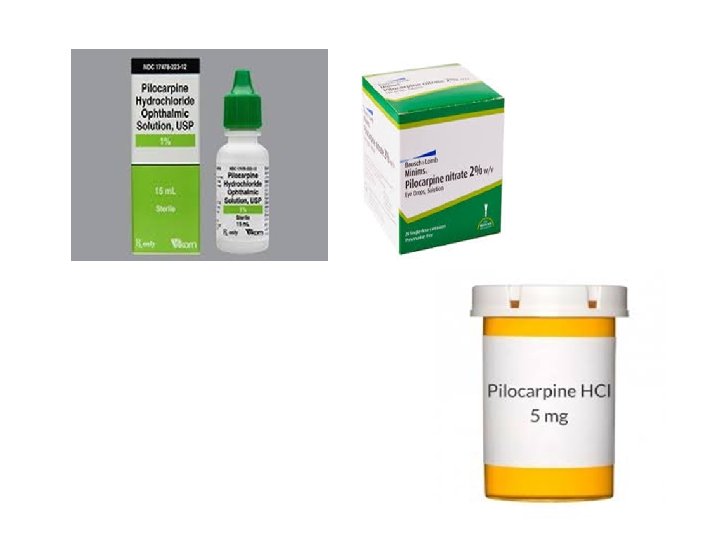
9. Imidazole Alkaloids • 1. Pilocarpine • (Pilocarpus spp). (ﺟﺒﻮﺭﻧﺪﻱ Rutaceae) Is a Cholinergic receptor agonist. It causes rapid miosis and contraction of the ciliary muscle in cornea and treatment of glaucoma treatment intraocular pressure(IOP). . Pilocarpine is an orally available cholinergic agonist that is used to treat symptoms of dry mouth (Xerostomia)also used to treat intraocular pressure(IOP).


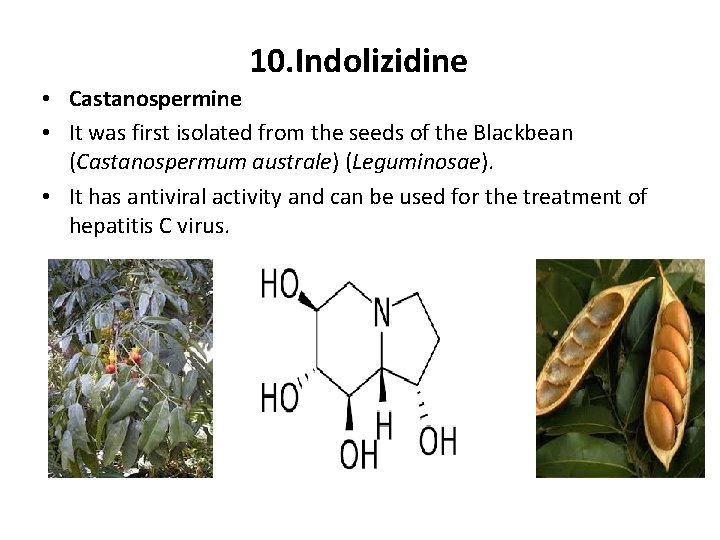
10. Indolizidine • Castanospermine • It was first isolated from the seeds of the Blackbean (Castanospermum australe) (Leguminosae). • It has antiviral activity and can be used for the treatment of hepatitis C virus.

11. Pyridine and piperidine • 1. Coniine? : Conium maculatum (Umbelliferae) • • 1. It has been used in convulsive and spasmodic diseases, such as: asthma, epilepsy, and tetanus. 2. Coniine has also been recommended is carditis, , glandular swellings, mania, nervous diseases, neuralgia, rheumatism, spasms and ulcers. • 2. Arecoline : Areca catechu (Arecaceae) • It is isolated from the seeds of the betel nut palm.

• Arecoline has a wide spectrum of pharmacological activities including effects on nervous, cardiovascular, digestive and endocrine systems and anti-parasitic effects. • It is an agonist at both muscarinic and nicotinic acetylcholine receptors. It is used in the form of various salts as a ganglionic stimulant, a parasympathomimetic, especially in veterinary practice. It has been used as a euphoriant.

• 3. Lobeline: Lobelia spp. (Lobeliaceae) • Uses ? • 4. Nicotine (pyridine + pyrrolidine) • Nicotiana tabacum and other spp. (Solanaceae) • The type of nicotine found in tobacco plants, comes from the nightshade family. Red peppers, eggplant, tomatoes, and potatoes are examples of the nightshade family.

• Nicotine is the principal pharmacologically active component of the tobacco plant Nicotiana tabacum, the primary source of smoking tobacco. It is now widely accepted that humans inhale tobacco smoke in order to experience the psychopharmacological properties of the nicotine present in the smoke and that a majority of habitual smokers become addicted to the drug. Nicotine has reinforcing properties which are thought to be central to its ability to cause dependence. Additionally, it enhances arousal ﻧﻬﻮﺽ and attention. It also increases information processing, especially of visual information, and shortens reaction times. Furthermore, nicotine might have anxiety-reducing (anxiolytic) properties and might heighten mood.

• Some studies suggest that nicotine may improve memory and concentration. • nicotine stimulating the adrenal glands, which results in the release of adrenaline. • adrenaline stimulates the body. There is an immediate release of glucose, as well as an increase in heart rate, breathing activity, and blood pressure. Nicotine also makes the pancreas produce less insulin, causing a slight increase in blood sugar or glucose.

• Indirectly, nicotine causes the release of dopamine in the pleasure and motivation areas of the brain. A similar effect occurs when people take heroin or cocaine. The drug user experiences a pleasurable sensation. • Consuming nicotine is also linked to raised alertness, euphoria, and a sensation of being relaxed.

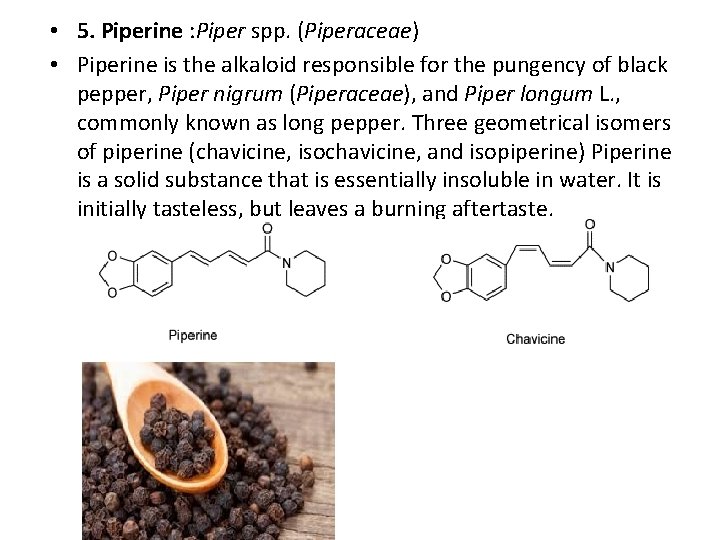
• 5. Piperine : Piper spp. (Piperaceae) • Piperine is the alkaloid responsible for the pungency of black pepper, Piper nigrum (Piperaceae), and Piper longum L. , commonly known as long pepper. Three geometrical isomers of piperine (chavicine, isochavicine, and isopiperine) Piperine is a solid substance that is essentially insoluble in water. It is initially tasteless, but leaves a burning aftertaste.

• The primary value of piperine in health supplements is its ability to enhance the bioavailability of some other vitamins and minerals. This mechanism is still being studied, but piperine is known to inhibit some enzymes in humans. Piperine has many pharmacological effects and several health benefits, especially against chronic diseases, such as reduction of insulin-resistance, anti-inflammatory effects, and improvement of hepatic steatosis. • It is also known that an increase in blood supply to the skin • Also it was used as antiepileptic drug in chinese medicine

• Ephedrine • Ephedra is the common name for three principal species: Ephedra sinica, Ephedra equisentina, and Ephedra intermedia. The active compounds in the plant’s stem (about 1. 32% by weight) are the phenylalanine-derived alkaloids ephedrine, pseudoephedrine, phenylpropanolamine (norephedrine), and cathine (norpseudoephedrine) • Ephedrine is agonist at α, β 1, and β 2 receptors with both direct and indirect actions.

• It causes peripheral constriction resulting in an increase in peripheral resistance that can lead to a sustained rise in blood pressure. It relaxes bronchial smooth muscle and is used as a decongestant and for temporary relief of shortness of breath caused by asthma • Ephedrine causes an increase in systolic, diastolic, and mean arterial pressures. It increases myocardial contractility, heart rate, and cardiac output.

• Colchicum autumnale(autumn crocus) • Colchicine is the major alkaloid from Colchicum autumnale L. and found also in other Colchicum species. Its primary therapeutic use is in the treatment of gout and prophylaxis of gout attack • Gout is an inflammatory arthritis that is the result of the precipitation of serum urate into crystallized deposits of monosodium urate (MSU) in and around the joint. • It causes depolymerization of tubulin, and it inhibits the production and release of leukotrienes.