Alkaloids introduction Pharmacognosy III Mosul University College of











- Slides: 17

Alkaloids introduction Pharmacognosy III Mosul University/ College of Pharmacy By: A. L. Noor Hisham Al-Atrakchi

ALKALOIDS

Definition • Typical alkaloids are basic nitrogenous compounds, usually of complex chemical structure , of plant origin and have a physiological activities. • ‘Proto-alkaloid’/ ‘amino-alkaloid’ – applied to compounds which lack one or more of the properties of typical alkaloids. • Other alkaloids, not confirming with the general definition, are those synthetic compounds not found in plants but very closely related to natural alkaloids (e. g. homatropine).

Occurrence& Distribution of Alkaloids • Alkaloids have a wide distribution, being present in plants, fungi, bacteria, amphibia, and animals. • True alkaloids are of rare occurrence in lower plants. • Occur in bacteria (Pseudomonas aeruginosa) and Ergotamine and ergometrine from ergot fungus. fungi , e. g. • Amphibians : Nearly 300 alkaloids belonging to more than 24 classes are known to occur in the skins of amphibians along with other toxins. They include the potent neurotoxic alkaloids of frog of genus Phyllobates, which are among the most poisonous substance known.

Physical and chemical properties • Most alkaloids are well-defined crystalline substances which unite with acids to form salts. • Most alkaloids are bitter in taste. • In rare cases they are colored. • In plants, they may exist in the free state, as salts or as N-oxides. • In addition to the elements carbon, hydrogen and nitrogen, most alkaloid contains oxygen. • A few such a s coniine from hemlock and nicotine from tobacco, are oxygen-free and are liquids. Ø Solubility • A knowledge of the solubility of alkaloids and their salts is of considerable pharmaceutical importance. the differences in solubility between alkaloids and their salts provides methods for the isolation of alkaloids from the plant and their separation from the non-alkaloidal substances also present.

Physical and chemical properties • The free bases are frequently sparingly soluble in water but soluble in organic solvents • Exceptions: caffeine and colchicine • With salts the reverse is often the case, these being usually soluble in water but sparingly soluble in organic solvents. • For example : strychnine hydrochloride is much more soluble in in water than strychnine base. • Exception : quinine sulphate is only soluble to the extent of 1 part in 1000 parts of water. • Alkaloids give a precipitate with heavy metal iodides. Exception: Caffeine, a purine derivative, does not precipitate like most alkaloids

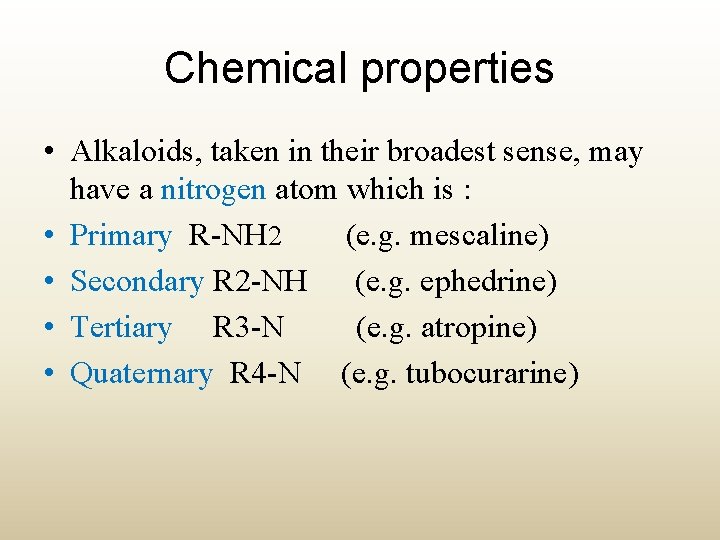
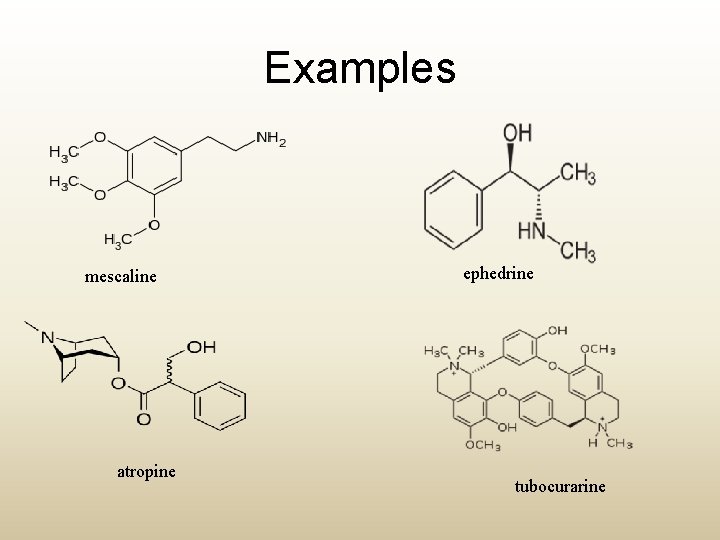
Chemical properties • Alkaloids, taken in their broadest sense, may have a nitrogen atom which is : • Primary R-NH 2 (e. g. mescaline) • Secondary R 2 -NH (e. g. ephedrine) • Tertiary R 3 -N (e. g. atropine) • Quaternary R 4 -N (e. g. tubocurarine)

Examples mescaline atropine ephedrine tubocurarine

Chemical properties • The degree of basicity varies considerably, depending on the structure of the molecule, and presence and location of the functional groups • The basicity of alkaloids depends on the availability of the pair of e- on the N 2 atoms: e- donating groups enhance basicity, while e-withdrawing groups decrease it.

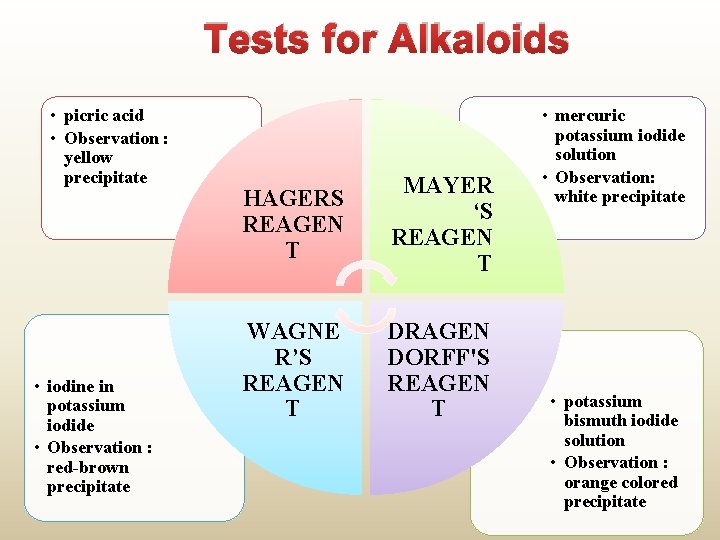
Tests for Alkaloids • picric acid • Observation : yellow precipitate • iodine in potassium iodide • Observation : red-brown precipitate HAGERS REAGEN T MAYER ‘S REAGEN T WAGNE R’S REAGEN T DRAGEN DORFF'S REAGEN T • mercuric potassium iodide solution • Observation: white precipitate • potassium bismuth iodide solution • Observation : orange colored precipitate

Naming of Alkaloids Numerous methods can be used to name alkaloids: • • • Generic plant name – atropine from Atropa belladonna Specific name of the plant – cocaine from coca. Common name – ergotamine from ergot Physiological action of the plant – emetine producing emesis Discoverer - e. g. Pelletierine from pelletier

Pharmacological Action & Uses The pharmacological action alkaloids varies widely: Ø CNS actions : Some alkaloids like (Morphine , codeine) are depressants used as analgesics and narcotics where as others ( strychnine , brucine, caffeine ) are central stimulants. Ø ANS: sympathomimetics (ephedrine) or sympatholytics ( ergot alkaloids), parasympathomimetics (pilocarpine). Ø Some alkaloids like (atropine) are mydriatics where as others (physostigmine, pilocarpine) are mitotic Ø some alkaloids like (ephedrine) cause arise blood pressure , other like (resepine) produce fall in excessive hypertension.

Pharmacological Action & Uses Other examples : Ø local anaesthetics (cocaine), Ø defibrillation (quinidine), Ø anti-tumour agents (vincristine), Ø anti-malarial (quinine), Ø anti-bacterials (berberine), These actions lead to the extensive use of alkaloid containing herbs and drugs. Although some are used as galenicals (belladonna, datura, and henbane), most are used as starting materials for industrial extraction (morphine from opium, and quinine from Cinchona bark

Biogenesis of Alkaloids • True alkaloids are based on an amino acid (pre-cursor). • Only a few amino acids form the pre-cursors for all alkaloids: ornithine, lysine, phenylalanine, tyrosine, tryptophan, histidine and anthranilic acid. • Alkaloid formation may require the involvement of: o only one molecule of amino acid, o or 2 molecules of the same AA, o or less commonly, 2 molecules of different AA o or several molecules of the same AA.


Structure and classification • Alkaloids show great variety in their botanical and biochemical origin, in chemical structure and in pharmacological actions. Consequently, many different systems of classification are possible. There are two broad divisions: • Non-heterocyclic or atypical alkaloids, • sometimes called proto-alkaloids or biological amines. • they are simple amines or biological amines , • contain nitrogen atom in the side chain, • basic in nature , • Examples : mescaline and ephedrine. • Heterocyclic or typical alkaloids, divided into 14 groups according to their ring structure.

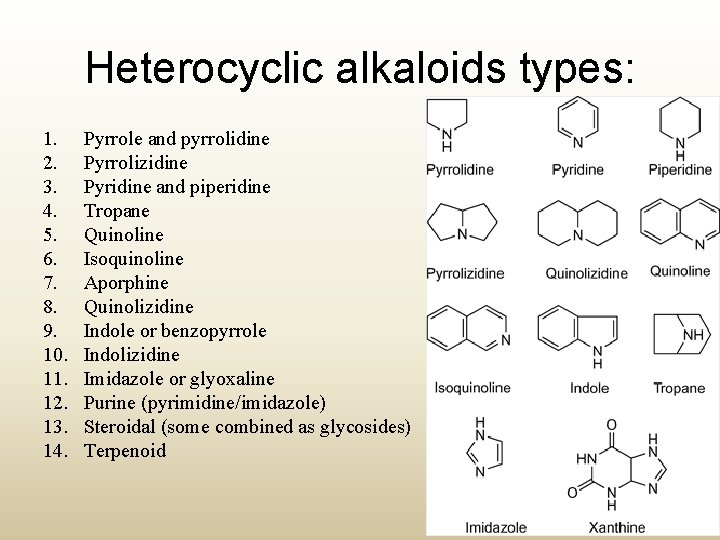
Heterocyclic alkaloids types: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. Pyrrole and pyrrolidine Pyrrolizidine Pyridine and piperidine Tropane Quinoline Isoquinoline Aporphine Quinolizidine Indole or benzopyrrole Indolizidine Imidazole or glyoxaline Purine (pyrimidine/imidazole) Steroidal (some combined as glycosides) Terpenoid
 Pharmacognosy alkaloids
Pharmacognosy alkaloids Norlupinane
Norlupinane Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Pharmacognosy father name
Pharmacognosy father name Hamlet act iii scene iii
Hamlet act iii scene iii Pyridine piperidine alkaloids
Pyridine piperidine alkaloids Ergoline alkaloids
Ergoline alkaloids Biological importance of alkaloids
Biological importance of alkaloids Ephedra
Ephedra Protoalkaloid
Protoalkaloid Purine alkaloids
Purine alkaloids Alkaloids derived from tyrosine
Alkaloids derived from tyrosine Purine alkaloids
Purine alkaloids Capsicum
Capsicum Solanacease
Solanacease Ergot alkaloids alpha blockers
Ergot alkaloids alpha blockers Tocolytic drugs
Tocolytic drugs






























