Unit 4 Different Types of Particles Day 1































- Slides: 61

Unit 4: Different Types of Particles

Day 1 Activity/Assignment Learning Targets • Discussion about the • Review particle Hindenburg knowledge • Explain why scientists are interested in different types of particles

• http: //www. youtube. com/watch? v=F 54 rq. Dh 2 m. WA What was the hydrogen gas used for? What is the problem with hydrogen gas? • What do we fill our balloons up with to get the same effect?

Why are blimps safe? Unlike the great German Zeppelins of fifty years ago, the Goodyear blimps are filled with helium, an inert gas. Although Hydrogen is a better lifting gas, lighter and more plentiful than helium, it is terribly flammable, even explosive. Helium is found in the earth, mixed with other natural gases. The most significant deposits yet discovered are in northern Texas, Kansas and Colorado.

Characteristics of Particles Compare the Goodyear blimp with the Hindenburg: • Differences? • Similarities?

Particles • Are in constant motion • Always move in straight lines • Transfer ENERGY by bumping into each other or their surroundings • Some particles are MOLECULES – made up of smaller particles

Molecules • Examples of molecules: – Water, salt, sugar, helium gas, carbon dioxide • The smaller particles that make up molecules are TIGHTLY bonded together – Sometimes when we break bonds, the reaction gives off energy – Sometimes when we break bonds, the reaction takes in energy

Day 2 Activity/Assignment Learning Targets • Un-mixing mixtures • Mystery Liquids • Describe characteristics that can be used to distinguish between substances and mixtures

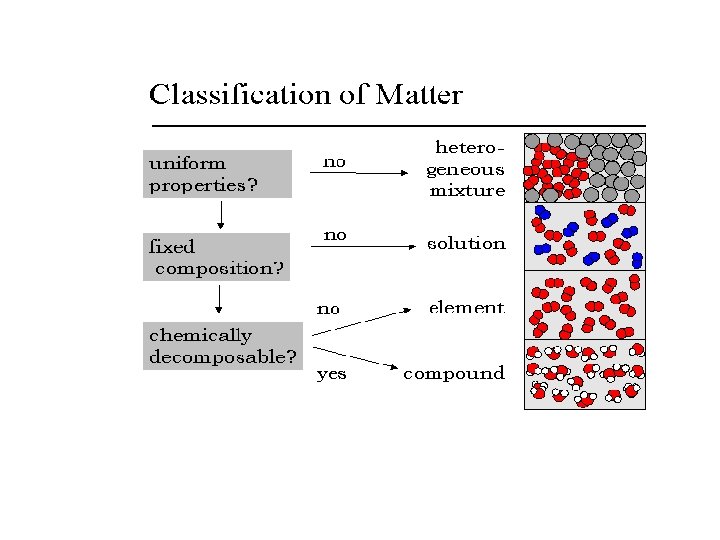
How do we know when something is a mixture?
How can we separate mixtures?

Mystery Liquids Clean-up • DO NOT pour liquids down the sink!!! • Carefully pour liquids in waste jars • Rinse out beakers and return all items to box • Return box to front of class

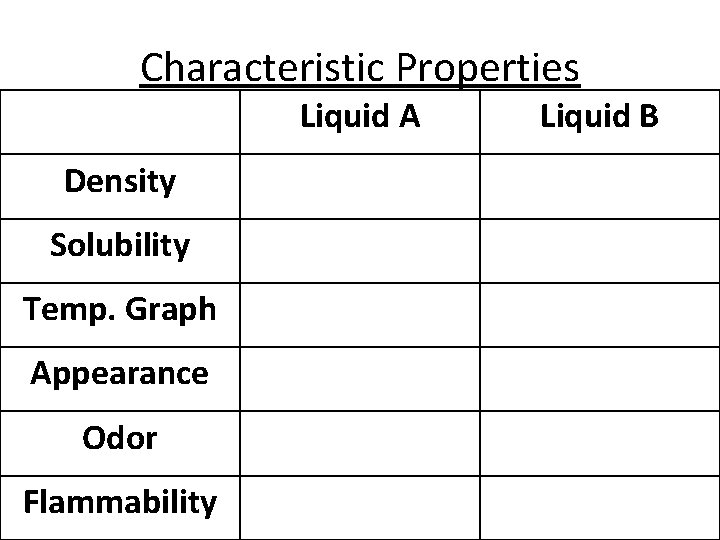
Characteristic Properties Liquid A Density Solubility Temp. Graph Appearance Odor Flammability Liquid B

How do we know when something is a mixture or a pure substance? ET: Record in your journal the answer to this question about lemonade: Is lemonade a mixture or a pure substance? Why?

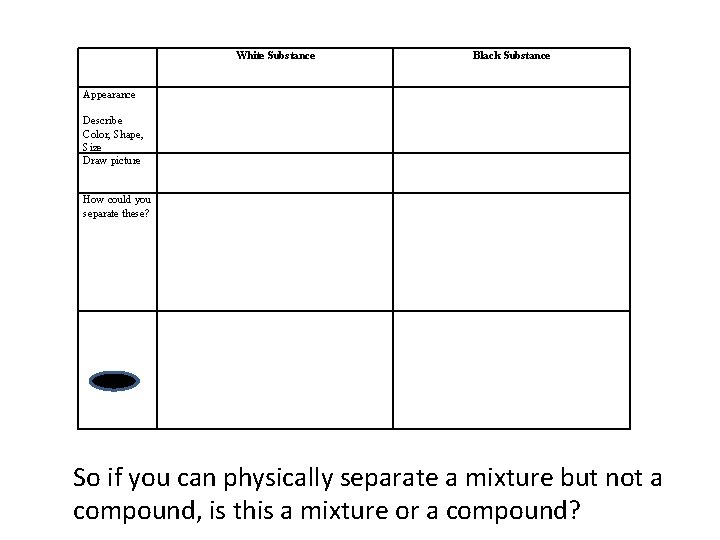
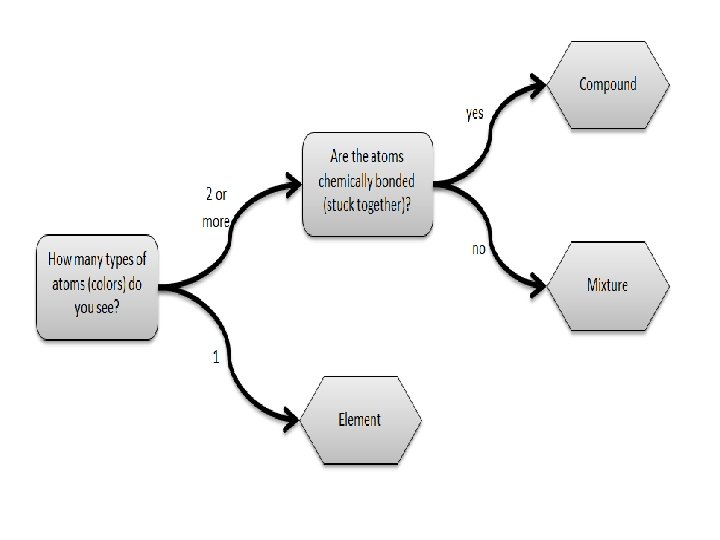
White Substance Black Substance Appearance Describe Color, Shape, Size Draw picture How could you separate these? So if you can physically separate a mixture but not a compound, is this a mixture or a compound?

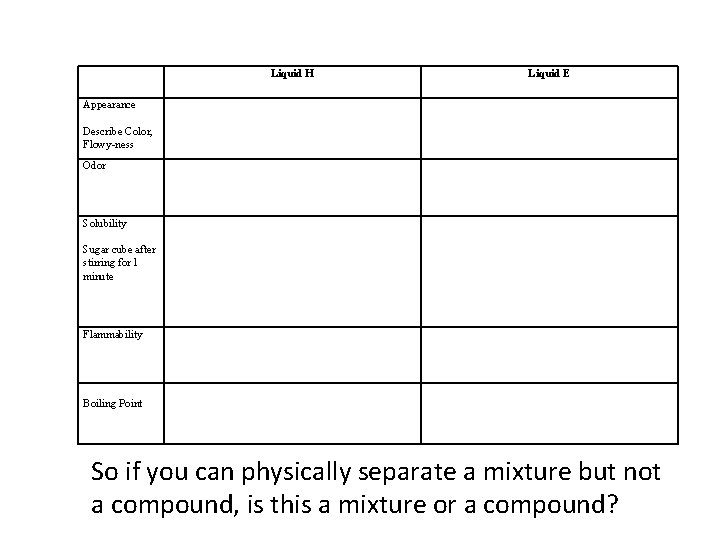
Liquid H Liquid E Appearance Describe Color, Flowy-ness Odor Solubility Sugar cube after stirring for 1 minute Flammability Boiling Point So if you can physically separate a mixture but not a compound, is this a mixture or a compound?


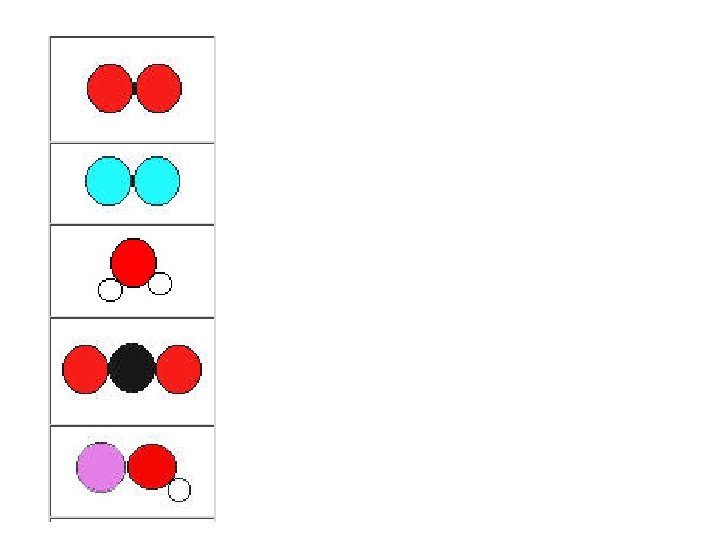
Day 3 (block) Activity/Assignment Learning Targets • Jelly bean atoms • Unit 4 Worksheet 1 • Distinguish between elements, compounds, and mixtures. Homework today: Elements, Compounds, and Mixtures Worksheet


• For each jelly bean combo answer these questions: –DRAW the beans you see. Remember these represent atoms. –Is this an element, compound or mixture? –How can you tell? (use flowchart) • After you are finished with all 6 stations, cover your answer sheet and have a partner quiz you. Your teacher will call you up to identify a set up at her table. You should get checked off before the end of the period.

Day 4 Activity/Assignment Learning Targets • Break apart water • (also: Go over classwork and homework; list new characteristic property) • Using water as an example, explain “fixed ratios” of atoms in compounds

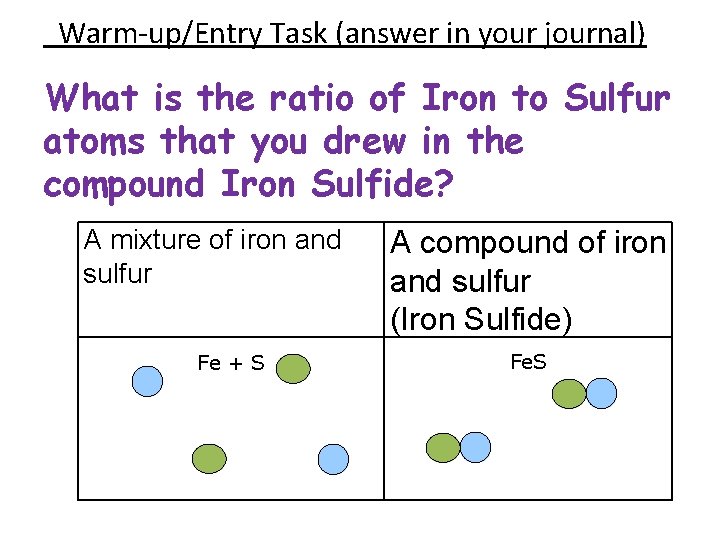
Warm-up/Entry Task (answer in your journal) What is the ratio of Iron to Sulfur atoms that you drew in the compound Iron Sulfide? A mixture of iron and sulfur Fe + S A compound of iron and sulfur (Iron Sulfide) Fe. S

Are all compounds in a fixed ratio? Are all compounds in a fixed ratio of 1: 1?

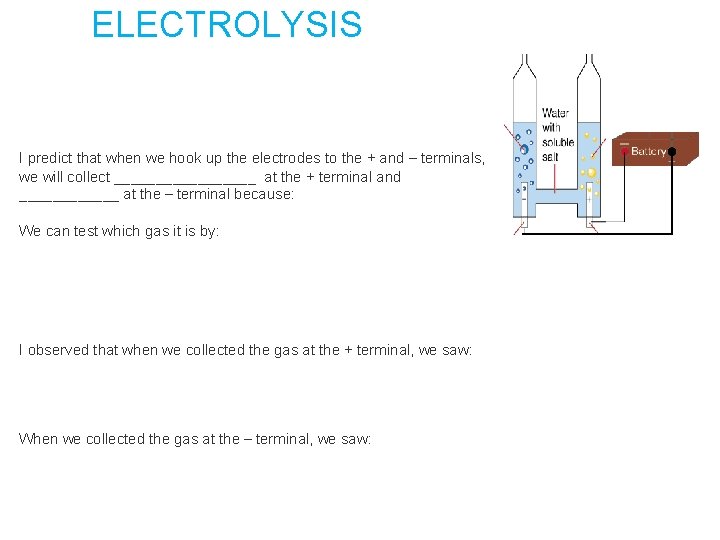
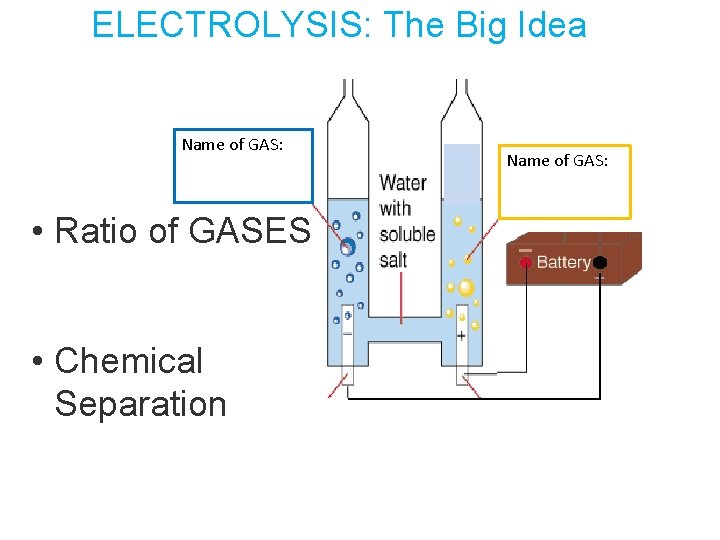
ELECTROLYSIS I predict that when we hook up the electrodes to the + and – terminals, we will collect _________ at the + terminal and ______ at the – terminal because: We can test which gas it is by: I observed that when we collected the gas at the + terminal, we saw: When we collected the gas at the – terminal, we saw:

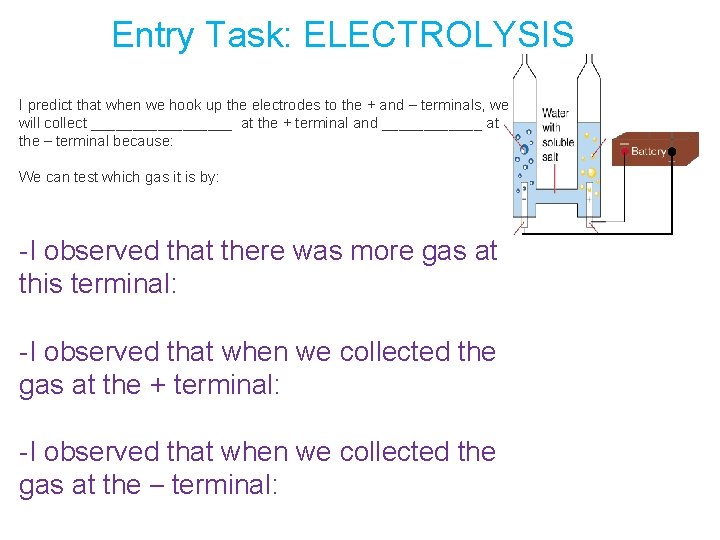
Entry Task: ELECTROLYSIS I predict that when we hook up the electrodes to the + and – terminals, we will collect _________ at the + terminal and ______ at the – terminal because: We can test which gas it is by: -I observed that there was more gas at this terminal: -I observed that when we collected the gas at the + terminal: -I observed that when we collected the gas at the – terminal:

Day 5 Activity/Assignment Learning Targets • Chemical Formulas Worksheet • Be able to write the chemical formulas for compounds and elements

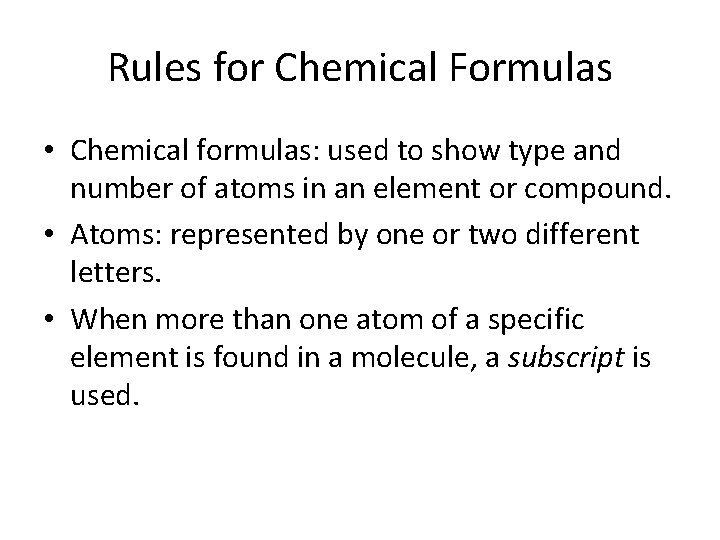
Rules for Chemical Formulas • Chemical formulas: used to show type and number of atoms in an element or compound. • Atoms: represented by one or two different letters. • When more than one atom of a specific element is found in a molecule, a subscript is used.

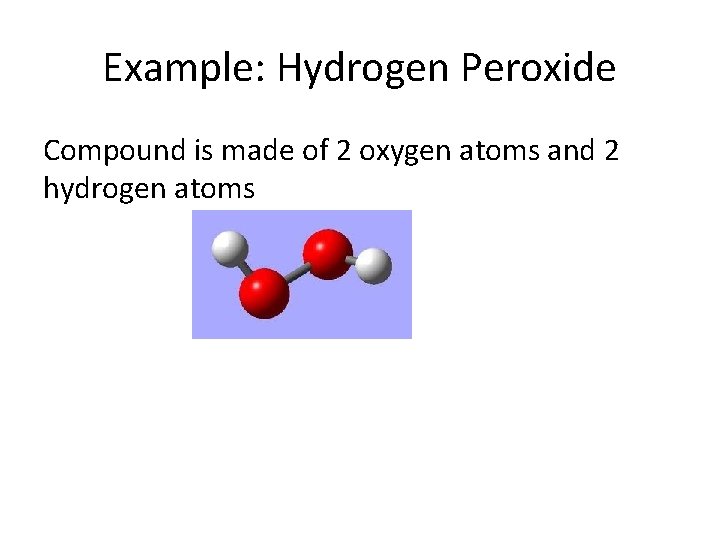
Example: Hydrogen Peroxide Compound is made of 2 oxygen atoms and 2 hydrogen atoms



Day 6 Activity/Assignment Learning Targets • Unit 4 Checkpoint Worksheet • Be able to write the chemical formulas for compounds and elements • Review elements, compounds, and mixtures

Day 7 (block) Activity/Assignment Learning Targets • Computer Lab: Dalton’s Playhouse • Explain experiments done by scientists that showed how elements and compounds combine or break apart in chemical reactions

Chemical Reactions Vocabulary • Reactants: Elements or compounds that are put in to a chemical reaction (the “ingredients”) • Products: Elements or compounds that are made from a chemical reaction (the “cake”)

Day 8 Activity/Assignment Learning Targets • Dalton’s Playhouse Whiteboards • Explain experiments done by scientists that showed how elements and compounds combine or break apart in chemical reactions

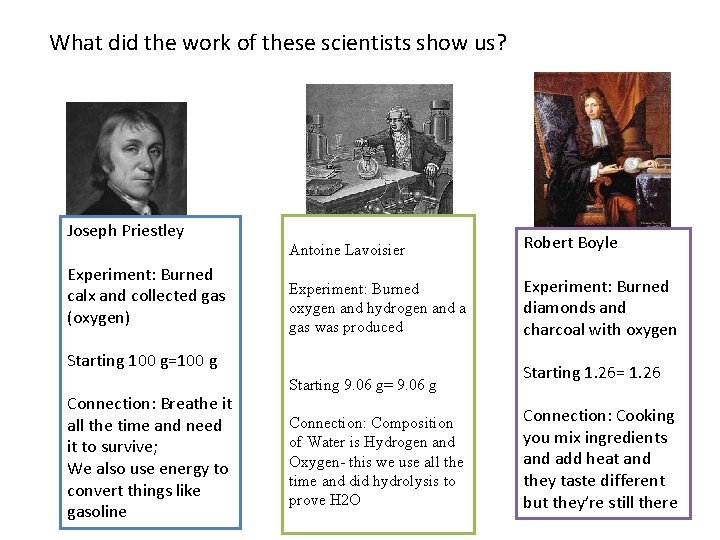
What did the work of these scientists show us? On your white board include: 1. Diagram of the Experiment (labels) 2. Evidence that starting mass = ending mass 3. One thing connected to our lives that came out of this experiment

Friday 4. 6: What did the work of these scientists show us? Take out your completed lab to be stamped Joseph Priestley Robert Boyle Antoine Lavoisier

Friday 4. 6: What did the work of these scientists show us? Take out your completed lab to be stamped Joseph Priestley Reactant= Calx Product=Oxygen 7. 39 lost=gained We use energy to convert OXYGEN Antoine Lavoisier Robert Boyle 4. 8 lost=gained Reactants= Diamond + Oxygen Hydrogen+ Oxygen= Water Products= Gas 1. 39 lost=gained

What did the work of these scientists show us? Joseph Priestley Experiment: Burned calx and collected gas (oxygen) Antoine Lavoisier Robert Boyle Experiment: Burned oxygen and hydrogen and a gas was produced Experiment: Burned diamonds and charcoal with oxygen Starting 100 g=100 g Connection: Breathe it all the time and need it to survive; We also use energy to convert things like gasoline Starting 9. 06 g= 9. 06 g Connection: Composition of Water is Hydrogen and Oxygen- this we use all the time and did hydrolysis to prove H 2 O Starting 1. 26= 1. 26 Connection: Cooking you mix ingredients and add heat and they taste different but they’re still there

Day 9 Activity/Assignment Learning Targets • Review for Quiz • Review for quiz

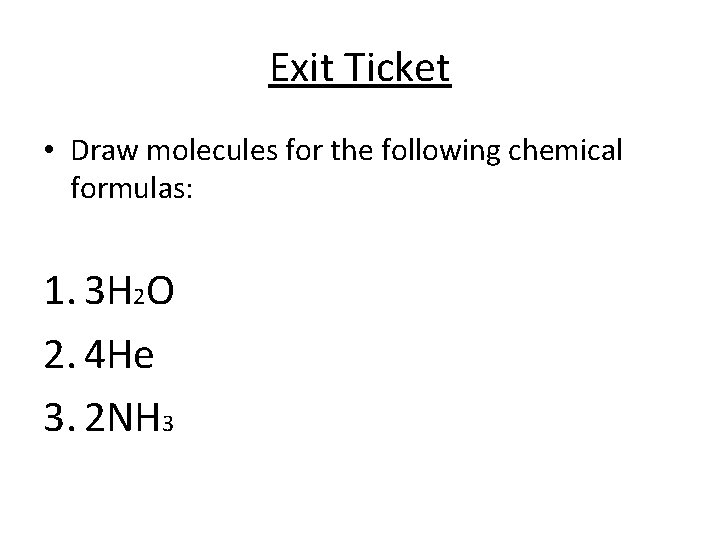
Exit Ticket • Draw molecules for the following chemical formulas: 1. 3 H 2 O 2. 4 He 3. 2 NH 3

Day 9 -10 Alternative Lessons

Now you are going to practice using evidence well. Record in your journal: I know that water is made of 2 parts Hydrogen and 1 part Oxygen because in class we : This shows that: If it weren’t true, that would mean that:

ELECTROLYSIS: The Big Idea Name of GAS: • Ratio of GASES • Chemical Separation Name of GAS:

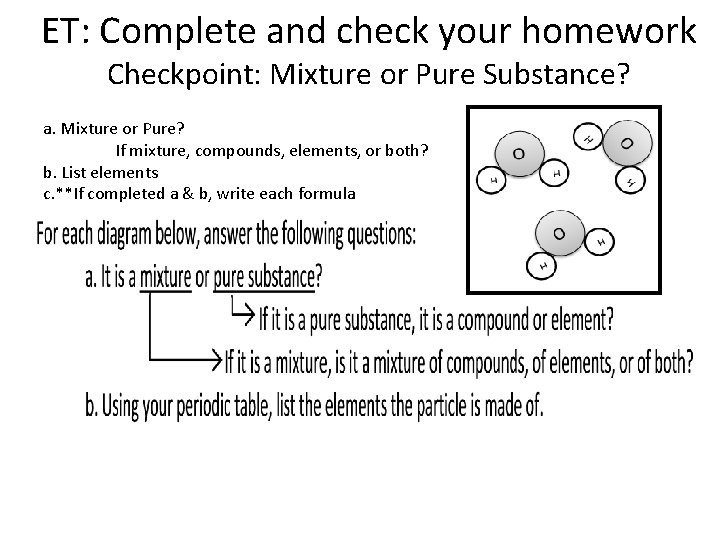
ET: Complete and check your homework Checkpoint: Mixture or Pure Substance? a. Mixture or Pure? If mixture, compounds, elements, or both? b. List elements c. **If completed a & b, write each formula

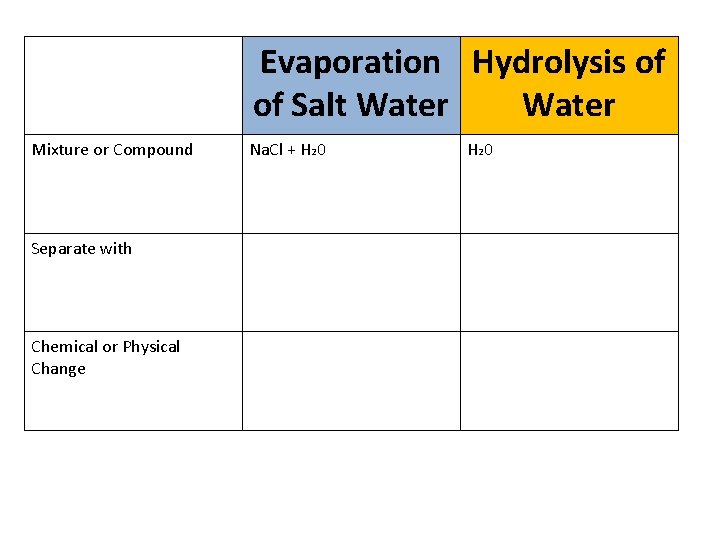
Evaporation Hydrolysis of of Salt Water Mixture or Compound Separate with Chemical or Physical Change Na. Cl + H 20 H 2 0

Can we use hydrolysis to solve energy issues? http: //www. zapkolik. com/59663/how-works-hydrolysis-simple-discovery. html

As drought and Global Warming intensify, conflict over fresh water will increase. Right now there are wars being fought over access to clean water. Democracy Now: http: //www. youtube. com/watch? v=amob. FCpe 83 Y Until 3: 30 or 6: 40 A Women’s Journey: http: //www. youtube. com/watch? v=Tc. XJn. ZBn. BRU&feature=related Until 1: 40 and then 4: 45 -10: 15

Answer in your journal Using the physical property of a mixture (you can separate salt from water), how do scientists use this knowledge to get fresh water from the ocean?

These countries have built what they think might be a technological solution to their water shortages ISRAEL AUSTRALIA

"In the past ten years, Victoria (Australia) has experienced several water crises, higher temperatures and record low rainfall. "

The plant site is about 500 meters inland associated infrastructure will include tunnels connecting the plant to marine intake and discharge structures up to 1. 2 km out to sea, an 85 kilometer pipeline to connect the plant to Melbourne's water supply system, and power supply infrastructure for the plant. Wonthaggi desalination plant Location Wonthaggi, AUSTRALIA Estimated output Extended output Cost 410 megalitres per day 550 megalitres per day A$3. 5 billion[1] Energy generation offset Windfarm at Glenthompson (proposed) Technology Percent of water supply Operation date Reverse Osmosis (proposed) DESALINIZATION Estimated 33% of Melbourne under construction

• Desalinization plant will pipe water 75 km away • Energy consumption is huge and the government proposes wind power to generate its electricity • Cost of project is est. $7. 5 US Billion at 2008 rates Nearby the desalination plant, Little Powlett River and oil rig… What is the environmental impact?

It is unquestionable that Australia is running out of fresh water and drastic action in needed. However opponents of the plan point to the huge energy requirements of seawater desalination, as well as the disruption of the aquatic and terrestrial environment by the plant and its pipelines.

They ask, “is there a better option? ” Why don’t we conserve our water? Take shorter showers, don’t let the water run while brushing teeth, reduce car washing and lawn watering, collect rainwater… Let’s be creative!

What do you think about these technological answers to energy and water problems? Record in your journal: What are some of the + and – you can think of about using these technologies to solve energy and water problems?

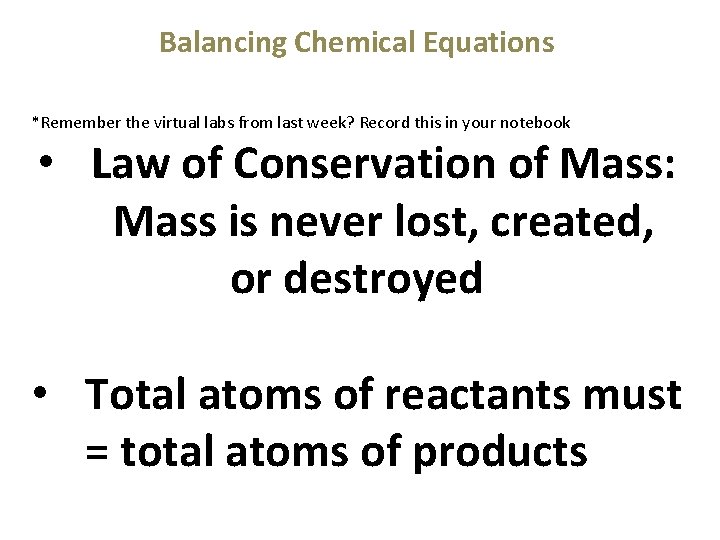
Balancing Chemical Equations *Remember the virtual labs from last week? Record this in your notebook • Law of Conservation of Mass: Mass is never lost, created, or destroyed • Total atoms of reactants must = total atoms of products

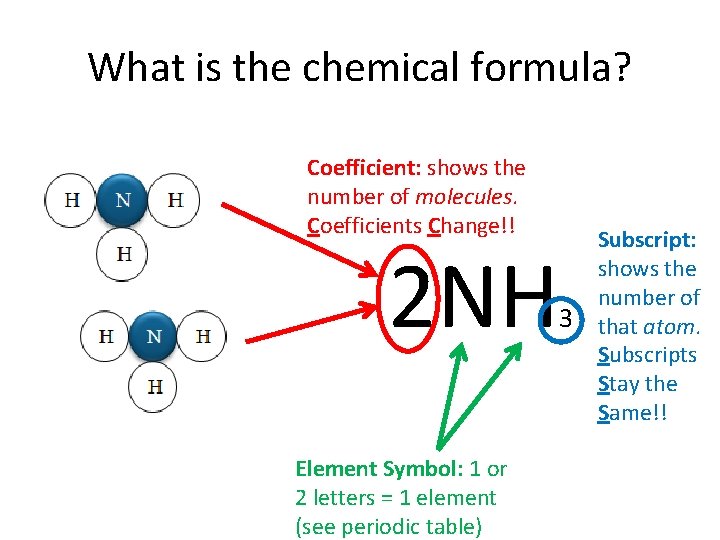
What is the chemical formula? Coefficient: shows the number of molecules. Coefficients Change!! 2 NH Element Symbol: 1 or 2 letters = 1 element (see periodic table) 3 Subscript: shows the number of that atom. Subscripts Stay the Same!!

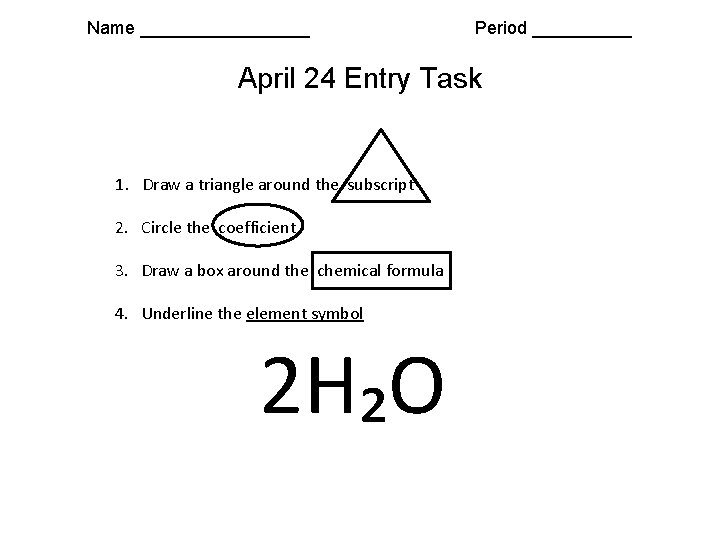
Name _________ Period _____ April 24 Entry Task 1. Draw a triangle around the subscript 2. Circle the coefficient 3. Draw a box around the chemical formula 4. Underline the element symbol 2 H₂O

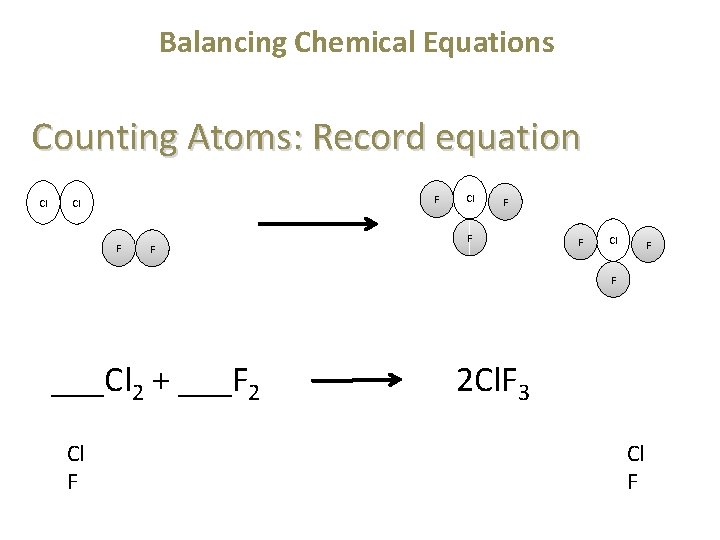
Balancing Chemical Equations Counting Atoms: Record equation Cl F F ___Cl 2 + ___F 2 2 Cl. F 3 Cl F Cl F

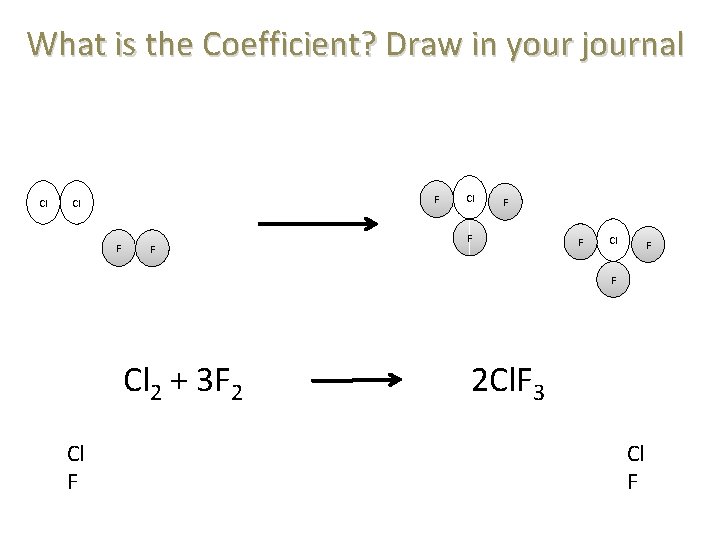
What is the Coefficient? Draw in your journal Cl F F Cl 2 + 3 F 2 2 Cl. F 3 Cl F Cl F

What are the Coefficients? Record in your journal ___CH 4 + O 2 ___CO 2 + ___H 2 O CH 4 + 2 O 2 CO 2 + 2 H 2 O ___O 2 + ___H 2 O O 2 + 2 H 2 O ___C + ___O 2 ___CO 2 C + O 2 CO 2 ___N 2 + ___O 2 ___NO N 2 + O 2 2 NO ___NO + ___O 2 ___NO 2 2 NO + O 2 2 NO 2 ___Na + ___H 2 O ___Na. OH + ___H 2 2 Na + 2 H 2 O 2 Na. OH + H 2 ___Cl 2 + ___F 2 ___Cl. F 3 Cl 2 + 3 F 2 2 Cl. F 3

Day 10 (block) Activity/Assignment Learning Targets • Quiz: Different Types of Particles