THE DIFFERENT TYPES OF THE DIFFERENT TYPES OF




- Slides: 15

THE DIFFERENT TYPES OF ___________________

THE DIFFERENT TYPES OF CHEMICAL REACTIONS

TODAY’S TARGETS: q. To be able to describe and identify 6 different types of Chemical Reactions

WHY DO WE CARE ABOUT CLASSIFYING TYPES OF CHEMICAL EQUATIONS? ? ? It is human nature to want to classify things. We classify music, food and. . . o Classifying allows us to PREDICT what will happen before the reaction occurs!

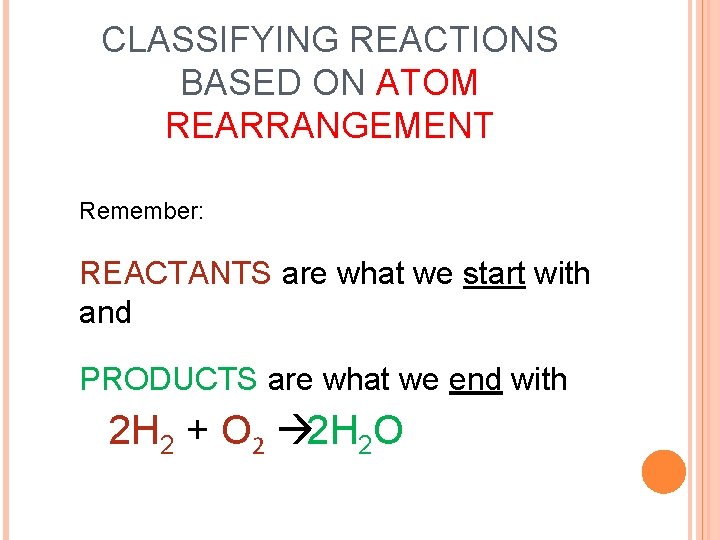
HOW DO WE CLASSIFY TYPES OF REACTIONS? Based on how the atoms become rearranged during the reaction.

CLASSIFYING REACTIONS BASED ON ATOM REARRANGEMENT Remember: REACTANTS are what we start with and PRODUCTS are what we end with 2 H 2 + O 2 à 2 H 2 O

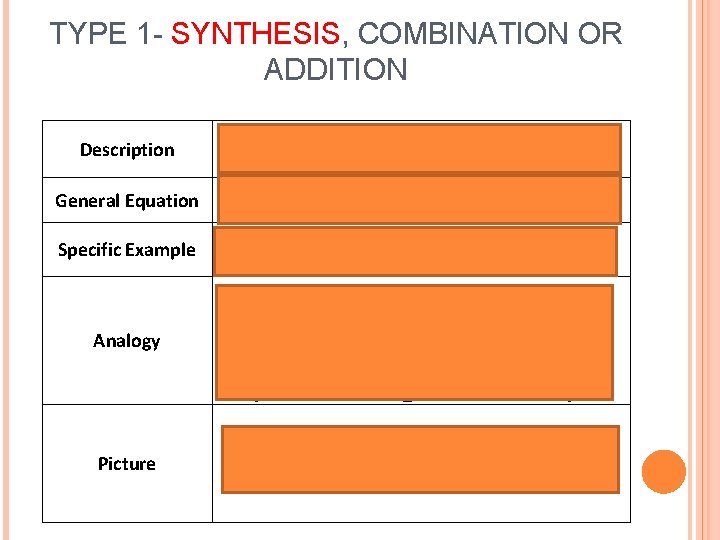
TYPE 1 - SYNTHESIS, COMBINATION OR ADDITION Description 2 or more reactants combine to make 1 single product General Equation A+ B àAB Specific Example 2 H 2 + O 2 à 2 H 2 O Analogy boy meets Picture girl = couple

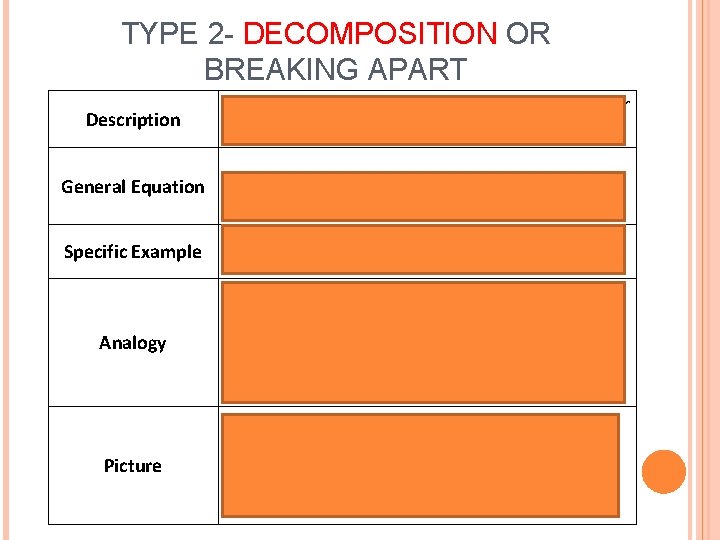
TYPE 2 - DECOMPOSITION OR BREAKING APART Description General Equation Specific Example 1 Single reactant is broken down into 2 or more products A BàA+ B 2 H 2 Oà 2 H 2 + O 2 Analogy The couple Breaks up! Picture

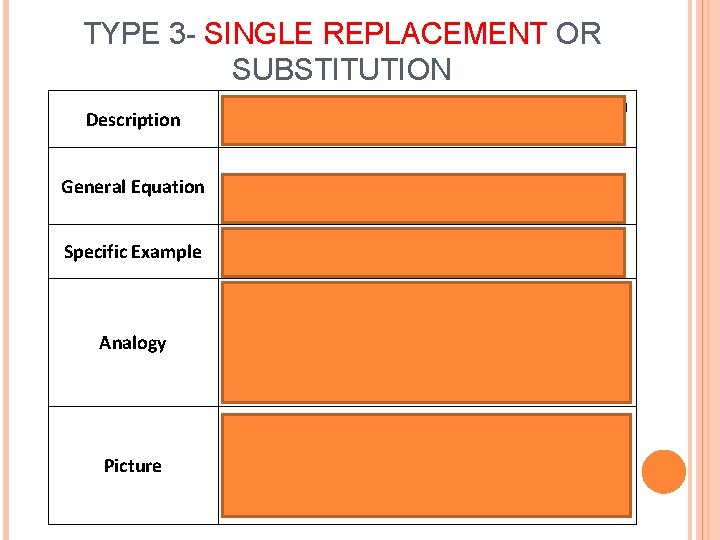
TYPE 3 - SINGLE REPLACEMENT OR SUBSTITUTION Description General Equation Specific Example 1 single element replaces an element in a compound A + BCàAC+ B Ca + Mg. Cl 2àCa. Cl 2 + Mg Analogy Like one girl switching dance partners Picture

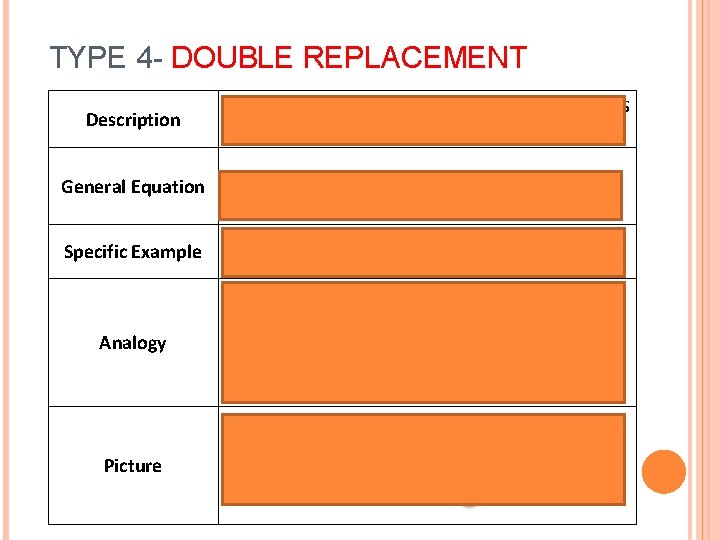
TYPE 4 - DOUBLE REPLACEMENT Description General Equation Specific Example When elements in 2 different compounds switch or change places AB + CDàAD+ CB Na. Cl + KIàNa. I + KCl Analogy Like 2 pairs or sets of dancers changing partners Picture

Double replacement reactions produce a precipitate. This is an insoluble solid (it doesn’t dissolve) that usually sinks to the bottom of the solution.

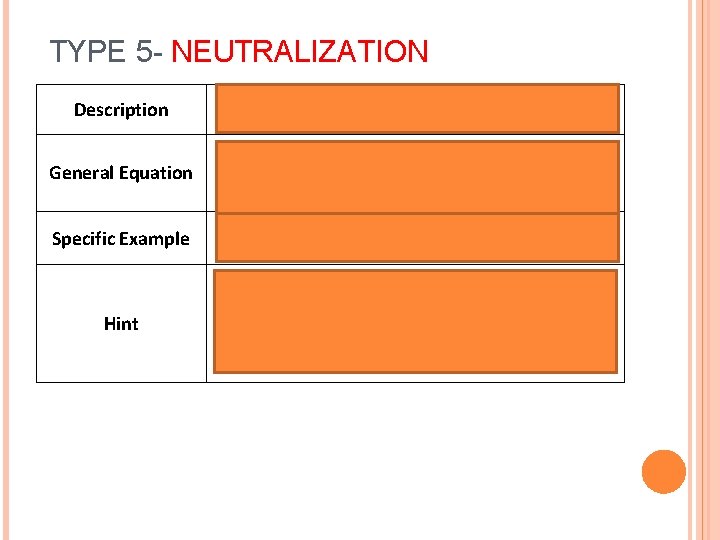
TYPE 5 - NEUTRALIZATION Description General Equation Specific Example Hint Acid + Base à Salt + Water HA + BOHàBA+ H 20 HCl + Na. OHàNa. Cl + H 2 O Will always have OH on reactant (left) side and H 20 on product (right) side

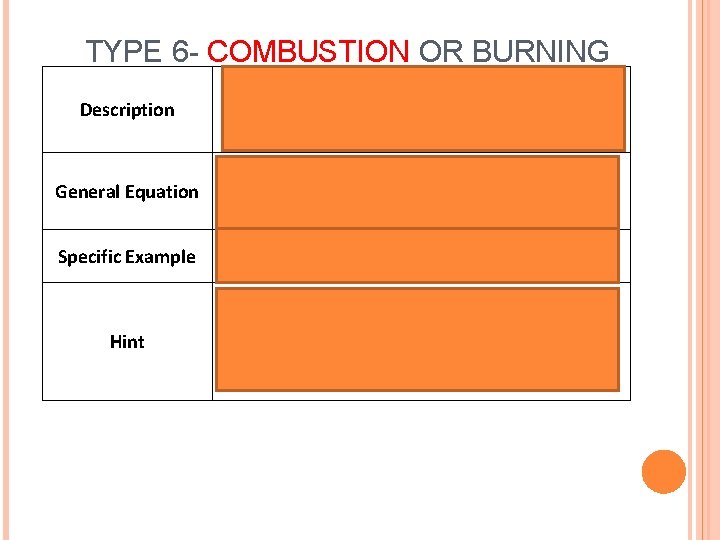
TYPE 6 - COMBUSTION OR BURNING Description General Equation Specific Example Hint Hydrocarbon reacts with Oxygen (O 2) to produce Heat, Carbon Dioxide (CO 2) and Water (H 2 O) Cx. Hy+ O 2àCO 2+ H 20 CH 4+ O 2àCO 2+ H 20 (methane) Will always have CO 2+ H 20 on product (right) side

REVIEW Atom rearrangement 1) Synthesis (Combination) 2) Decomposition 3) Single Replacement (Substitution) 4) Double Replacement 5) Neutralization 6) Combustion ( Burning)

LAST NOTE: Classifying chemical reactions is all about PATTERNS!!!