Topic 1 2 The VaporCompression Cycle The vaporcompression
- Slides: 30

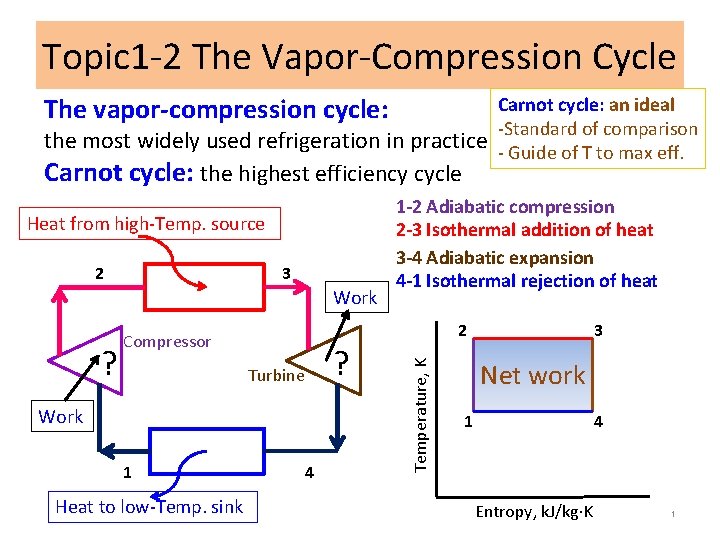
Topic 1 -2 The Vapor-Compression Cycle The vapor-compression cycle: the most widely used refrigeration in practice Carnot cycle: the highest efficiency cycle Heat from high-Temp. source 3 Work ? 2 Compressor ? Turbine Work 1 Heat to low-Temp. sink 1 -2 Adiabatic compression 2 -3 Isothermal addition of heat 3 -4 Adiabatic expansion 4 -1 Isothermal rejection of heat 4 Temperature, K 2 Carnot cycle: an ideal -Standard of comparison - Guide of T to max eff. 3 Net work 1 4 Entropy, k. J/kg K 1

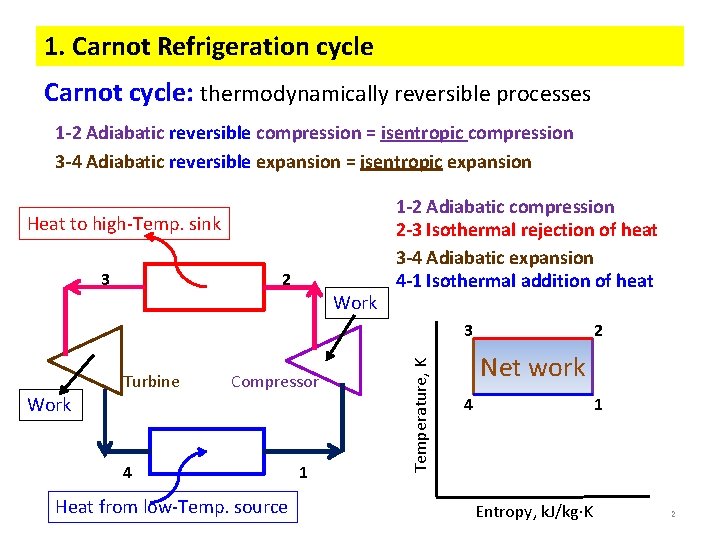
1. Carnot Refrigeration cycle Carnot cycle: thermodynamically reversible processes 1 -2 Adiabatic reversible compression = isentropic compression 3 -4 Adiabatic reversible expansion = isentropic expansion Heat to high-Temp. sink 3 2 Work 1 -2 Adiabatic compression 2 -3 Isothermal rejection of heat 3 -4 Adiabatic expansion 4 -1 Isothermal addition of heat Turbine Compressor Work 4 Heat from low-Temp. source 1 Temperature, K 3 2 Net work 4 1 Entropy, k. J/kg K 2

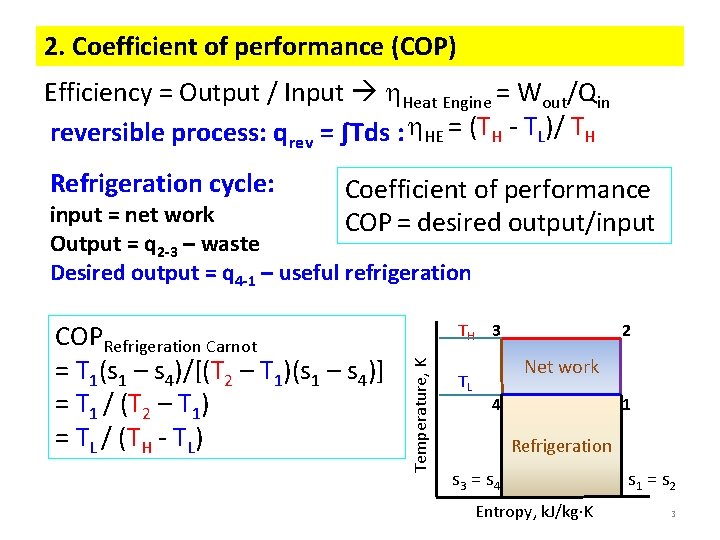
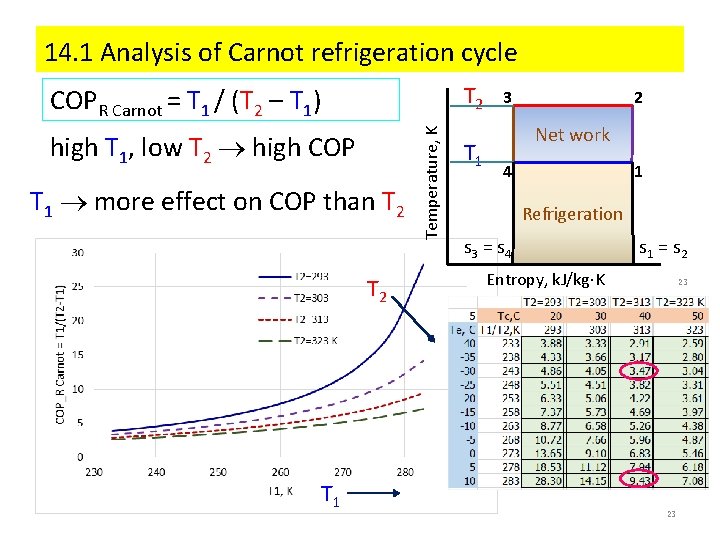
2. Coefficient of performance (COP) Efficiency = Output / Input Heat Engine = Wout/Qin reversible process: qrev = ∫Tds : HE = (TH - TL)/ TH Refrigeration cycle: Coefficient of performance COP = desired output/input = net work Output = q 2 -3 – waste Desired output = q 4 -1 – useful refrigeration TH 3 Temperature, K COPRefrigeration Carnot = T 1(s 1 – s 4)/[(T 2 – T 1)(s 1 – s 4)] = T 1 / (T 2 – T 1) = TL / (TH - TL) TL 2 Net work 4 1 Refrigeration s 3 = s 4 Entropy, k. J/kg K s 1 = s 2 3

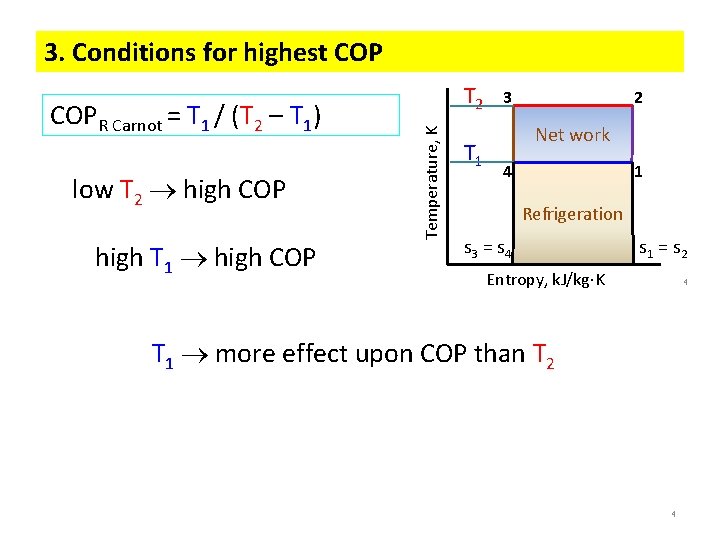
3. Conditions for highest COP low T 2 high COP high T 1 high COP Temperature, K COPR Carnot = T 1 / (T 2 – T 1) T 2 T 1 3 2 Net work 4 1 Refrigeration s 3 = s 4 s 1 = s 2 Entropy, k. J/kg K 4 T 1 more effect upon COP than T 2 4

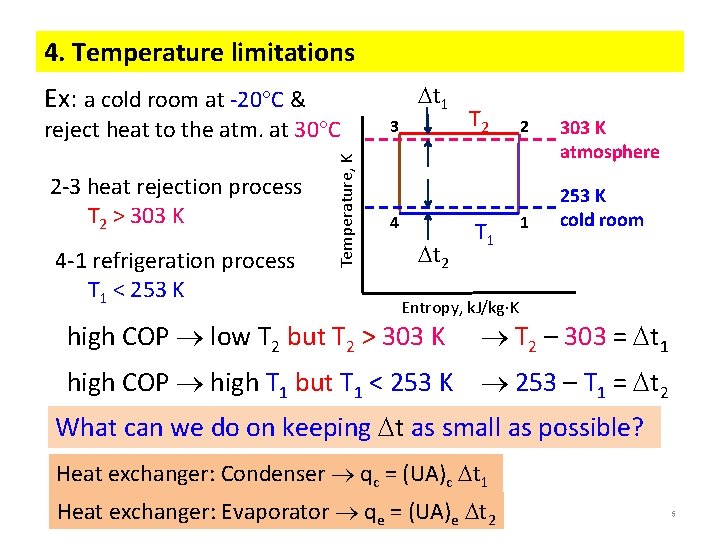
4. Temperature limitations t 1 Ex: a cold room at -20 C & 2 -3 heat rejection process T 2 > 303 K 4 -1 refrigeration process T 1 < 253 K Temperature, K reject heat to the atm. at 30 C 3 4 t 2 T 1 2 1 303 K atmosphere 253 K cold room Entropy, k. J/kg K high COP low T 2 but T 2 > 303 K T 2 – 303 = t 1 high COP high T 1 but T 1 < 253 K 253 – T 1 = t 2 What can we do on keeping t as small as possible? Heat exchanger: Condenser qc = (UA)c t 1 Heat exchanger: Evaporator qe = (UA)e t 2 5

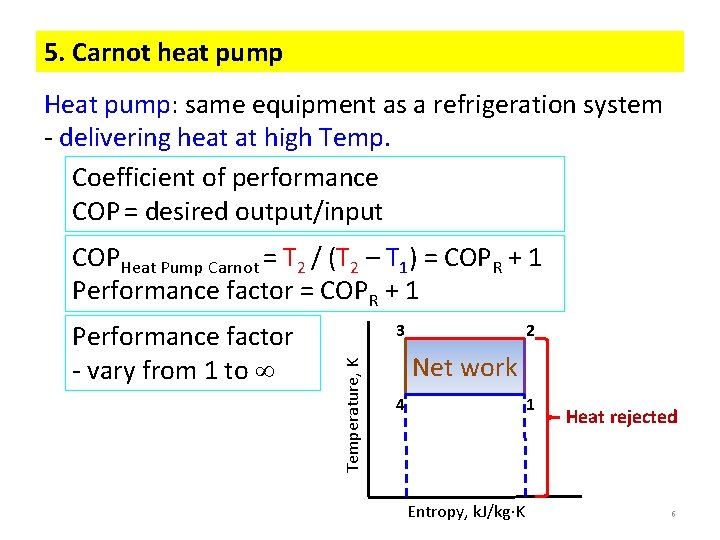
5. Carnot heat pump Heat pump: same equipment as a refrigeration system - delivering heat at high Temp. Coefficient of performance COP = desired output/input COPHeat Pump Carnot = T 2 / (T 2 – T 1) = COPR + 1 Performance factor = COPR + 1 3 Temperature, K Performance factor - vary from 1 to 2 Net work 4 1 Entropy, k. J/kg K Heat rejected 6

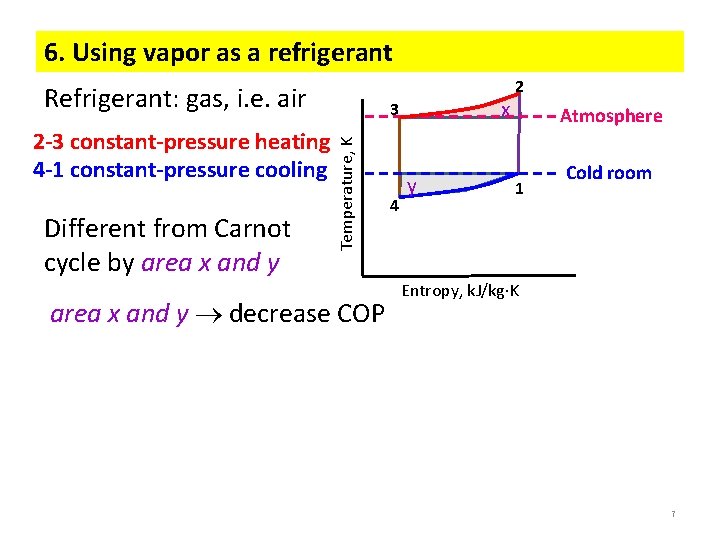
6. Using vapor as a refrigerant Refrigerant: gas, i. e. air Different from Carnot cycle by area x and y Temperature, K 2 -3 constant-pressure heating 4 -1 constant-pressure cooling x 3 area x and y decrease COP 4 y 2 Atmosphere 1 Cold room Entropy, k. J/kg K 7

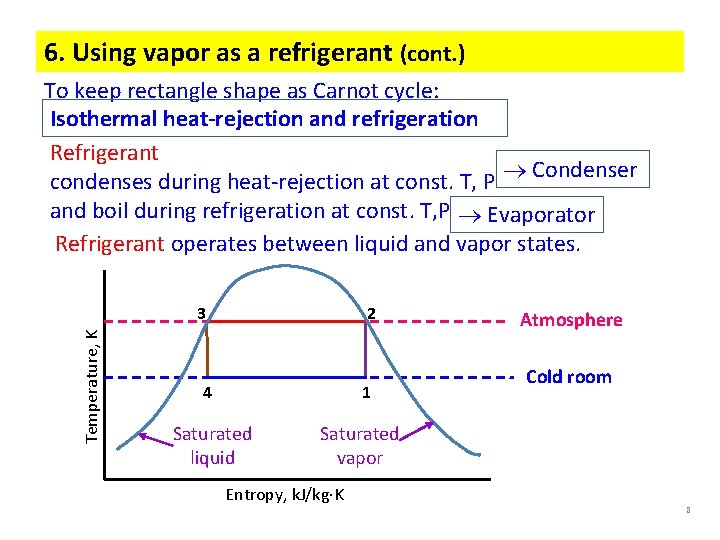
6. Using vapor as a refrigerant (cont. ) To keep rectangle shape as Carnot cycle: Isothermal heat-rejection and refrigeration Refrigerant condenses during heat-rejection at const. T, P Condenser and boil during refrigeration at const. T, P Evaporator Refrigerant operates between liquid and vapor states. Temperature, K 3 2 4 1 Saturated liquid Atmosphere Cold room Saturated vapor Entropy, k. J/kg K 8

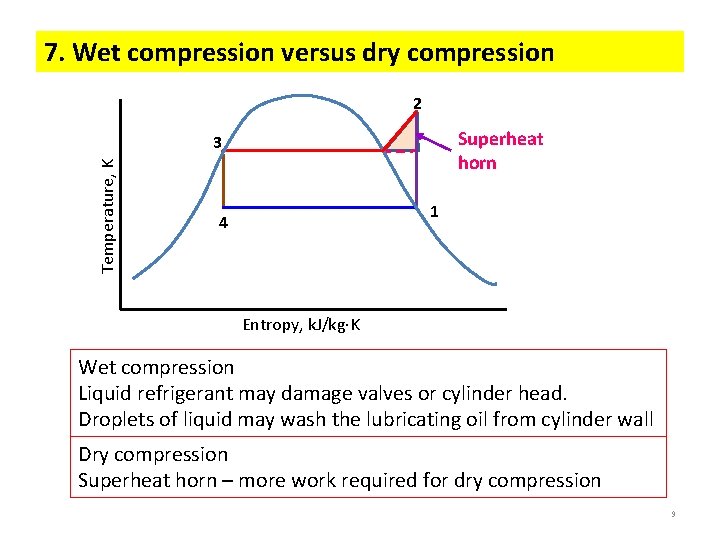
7. Wet compression versus dry compression 2 Superheat horn Temperature, K 3 1 4 Entropy, k. J/kg K Wet compression Liquid refrigerant may damage valves or cylinder head. Droplets of liquid may wash the lubricating oil from cylinder wall Dry compression Superheat horn – more work required for dry compression 9

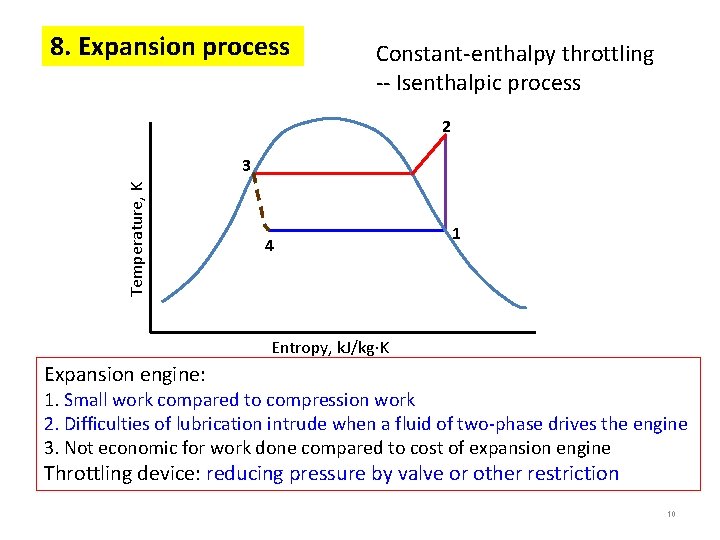
8. Expansion process Constant-enthalpy throttling -- Isenthalpic process 2 Temperature, K 3 4 1 Entropy, k. J/kg K Expansion engine: 1. Small work compared to compression work 2. Difficulties of lubrication intrude when a fluid of two-phase drives the engine 3. Not economic for work done compared to cost of expansion engine Throttling device: reducing pressure by valve or other restriction 10

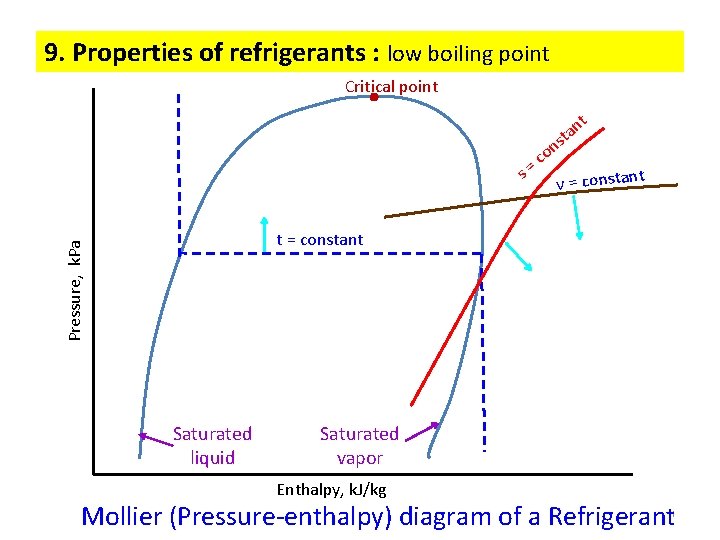
9. Properties of refrigerants : low boiling point Critical point t s o c = n sta n t v = constan Pressure, k. Pa t = constant Saturated liquid Saturated vapor Enthalpy, k. J/kg Mollier (Pressure-enthalpy) diagram of a Refrigerant 11

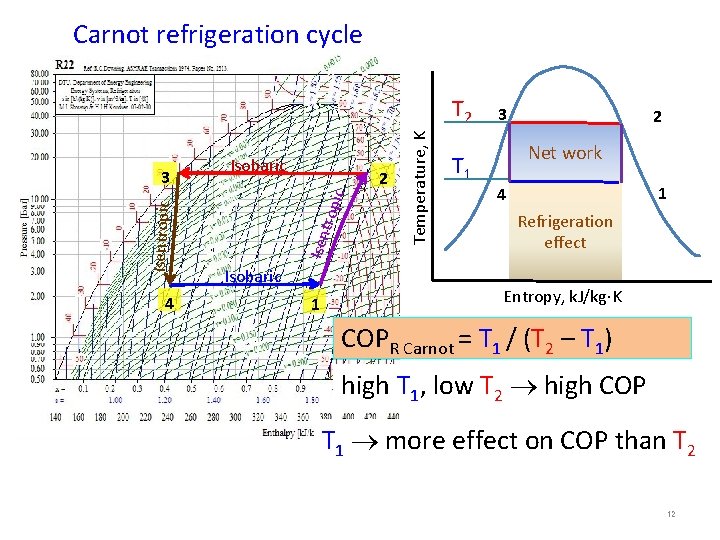
Carnot refrigeration cycle 4 trop ic 2 Isentropic 3 Isobaric 1 Temperature, K T 2 T 1 3 2 Net work 1 4 Refrigeration effect Entropy, k. J/kg K COPR Carnot = T 1 / (T 2 – T 1) high T 1, low T 2 high COP T 1 more effect on COP than T 2 12

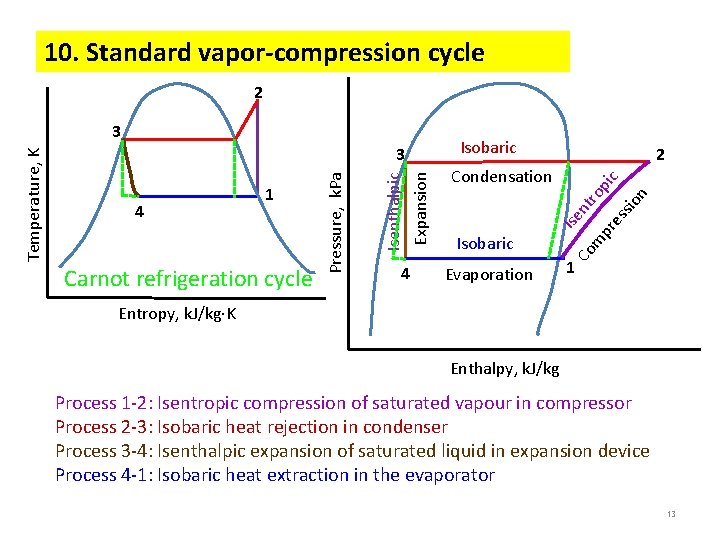
10. Standard vapor-compression cycle 2 Carnot refrigeration cycle 2 Evaporation n io ss m Isobaric pr e nt ro pi c Condensation Co 4 Isobaric Ise 4 1 Isenthalpic Expansion 3 Pressure, k. Pa Temperature, K 3 1 Entropy, k. J/kg K Enthalpy, k. J/kg Process 1 -2: Isentropic compression of saturated vapour in compressor Process 2 -3: Isobaric heat rejection in condenser Process 3 -4: Isenthalpic expansion of saturated liquid in expansion device Process 4 -1: Isobaric heat extraction in the evaporator 13

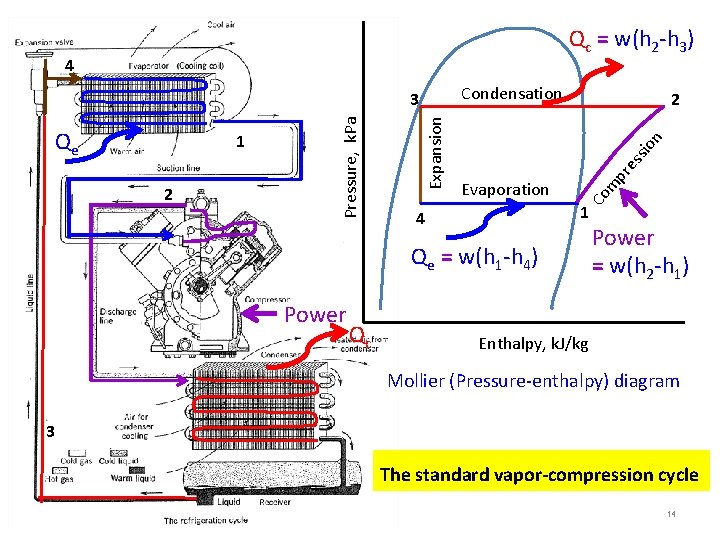
Qc = w(h 2 -h 3) 4 Condensation n io ss pr e Evaporation m 2 2 Co 1 Expansion Qe Pressure, k. Pa 3 1 4 Qe = w(h 1 -h 4) Power Qc Power = w(h 2 -h 1) Enthalpy, k. J/kg Mollier (Pressure-enthalpy) diagram 3 The standard vapor-compression cycle 14

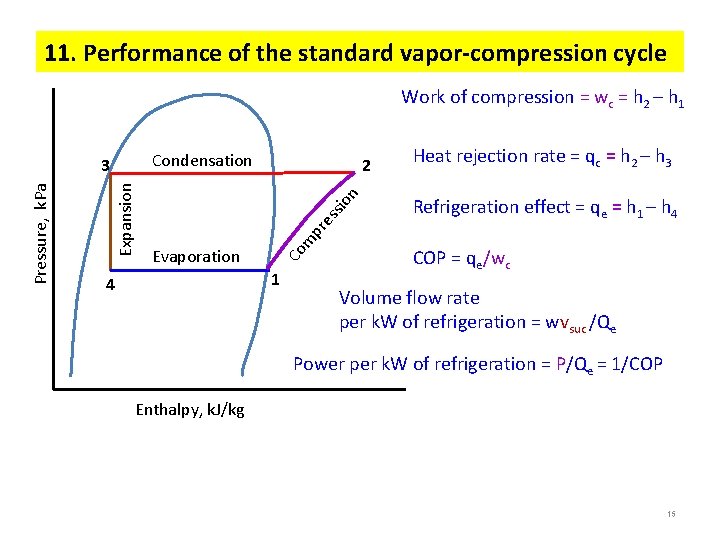
11. Performance of the standard vapor-compression cycle Work of compression = wc = h 2 – h 1 Condensation Heat rejection rate = qc = h 2 – h 3 Refrigeration effect = qe = h 1 – h 4 Evaporation m pr es sio n 2 Co Expansion Pressure, k. Pa 3 1 4 COP = qe/wc Volume flow rate per k. W of refrigeration = wvsuc /Qe Power per k. W of refrigeration = P/Qe = 1/COP Enthalpy, k. J/kg 15

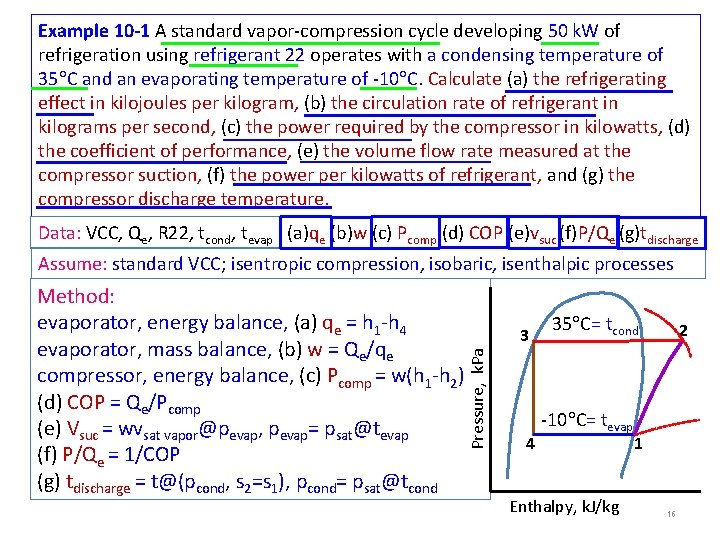
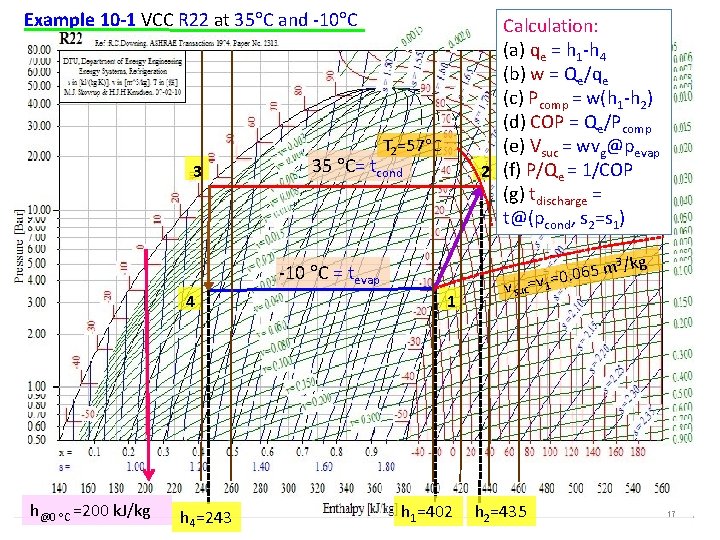
Example 10 -1 A standard vapor-compression cycle developing 50 k. W of refrigeration using refrigerant 22 operates with a condensing temperature of 35 C and an evaporating temperature of -10 C. Calculate (a) the refrigerating effect in kilojoules per kilogram, (b) the circulation rate of refrigerant in kilograms per second, (c) the power required by the compressor in kilowatts, (d) the coefficient of performance, (e) the volume flow rate measured at the compressor suction, (f) the power per kilowatts of refrigerant, and (g) the compressor discharge temperature. Data: VCC, Qe, R 22, tcond, tevap (a)qe (b)w (c) Pcomp (d) COP (e)vsuc (f)P/Qe (g)tdischarge Assume: standard VCC; isentropic compression, isobaric, isenthalpic processes 3 Pressure, k. Pa Method: evaporator, energy balance, (a) qe = h 1 -h 4 evaporator, mass balance, (b) w = Qe/qe compressor, energy balance, (c) Pcomp = w(h 1 -h 2) (d) COP = Qe/Pcomp (e) Vsuc = wvsat vapor@pevap, pevap= psat@tevap (f) P/Qe = 1/COP (g) tdischarge = t@(pcond, s 2=s 1), pcond= psat@tcond 4 35 C= tcond -10 C= tevap Enthalpy, k. J/kg 2 1 16

Example 10 -1 VCC R 22 at 35 C and -10 C Calculation: (a) qe = h 1 -h 4 (b) w = Qe/qe (c) Pcomp = w(h 1 -h 2) (d) COP = Qe/Pcomp (e) Vsuc = wvg@pevap 2 (f) P/Qe = 1/COP (g) tdischarge = t@(pcond, s 2=s 1) T 2=57 C 3 35 C= tcond -10 C = tevap 4 h@0 C =200 k. J/kg h 4=243 1 h 1=402 3 /kg m 5 6 0. 0 v suc=v 1= h 2=435 17

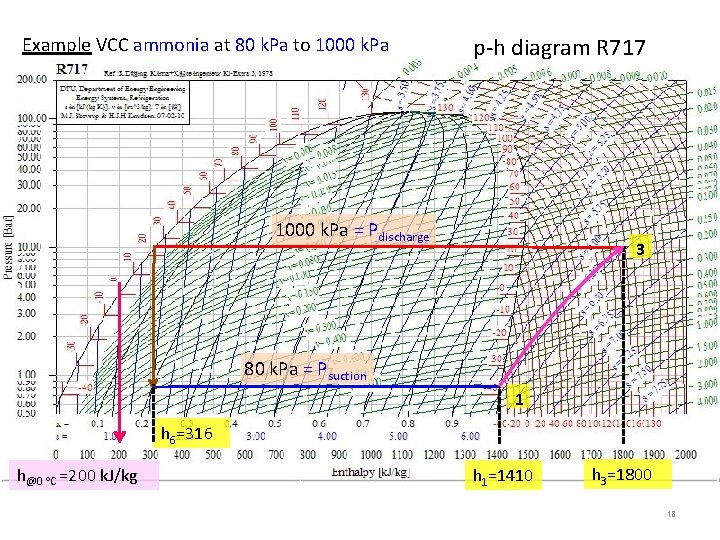
Example VCC ammonia at 80 k. Pa to 1000 k. Pa p-h diagram R 717 1000 k. Pa = Pdischarge 3 80 k. Pa = Psuction 1 h 6=316 h@0 C =200 k. J/kg h 1=1410 h 3=1800 18

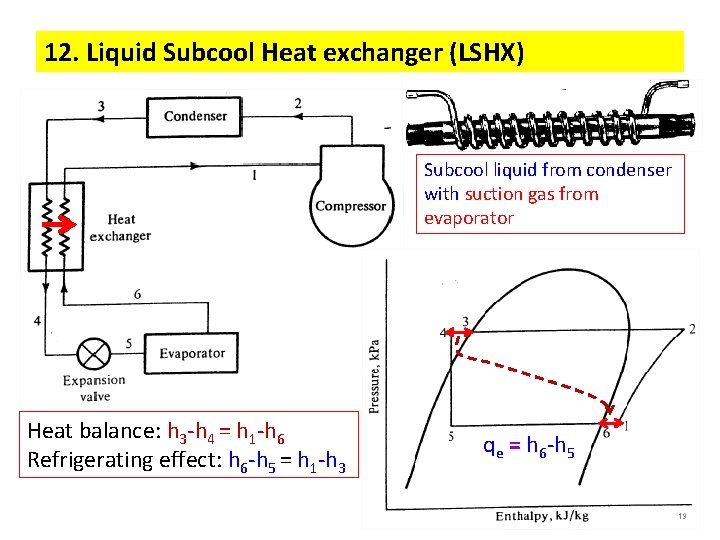
12. Liquid Subcool Heat exchanger (LSHX) Subcool liquid from condenser with suction gas from evaporator Heat balance: h 3 -h 4 = h 1 -h 6 Refrigerating effect: h 6 -h 5 = h 1 -h 3 qe = h 6 -h 5 19 19

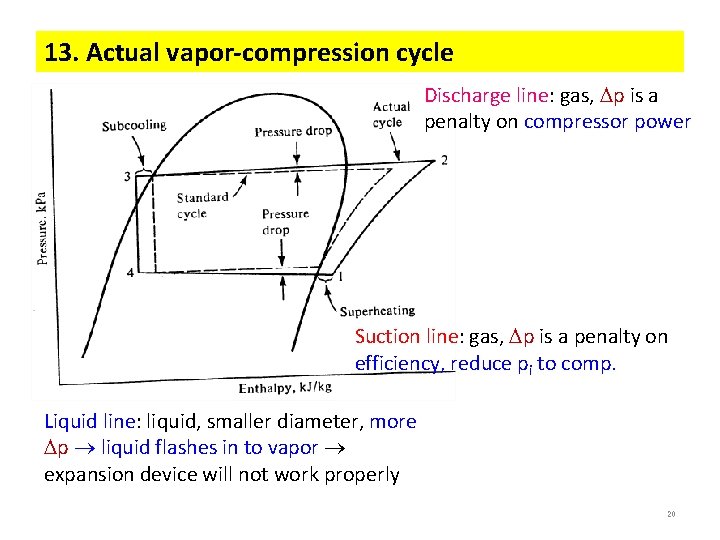
13. Actual vapor-compression cycle Discharge line: gas, p is a penalty on compressor power Suction line: gas, p is a penalty on efficiency, reduce pi to comp. Liquid line: liquid, smaller diameter, more p liquid flashes in to vapor expansion device will not work properly 20

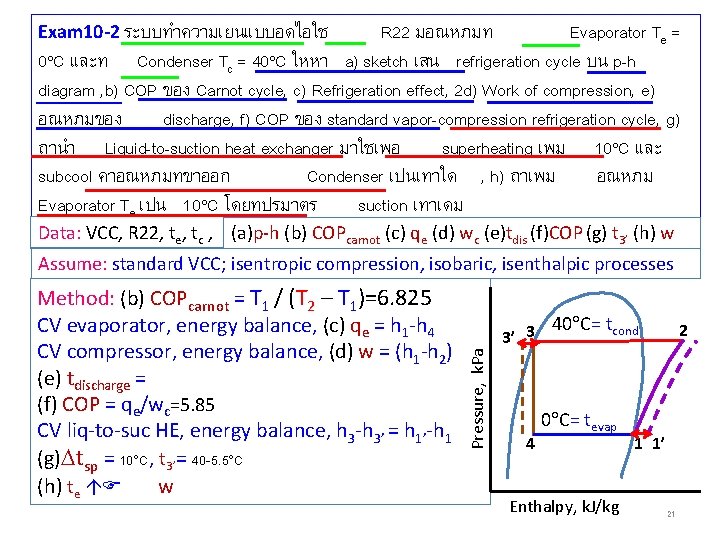
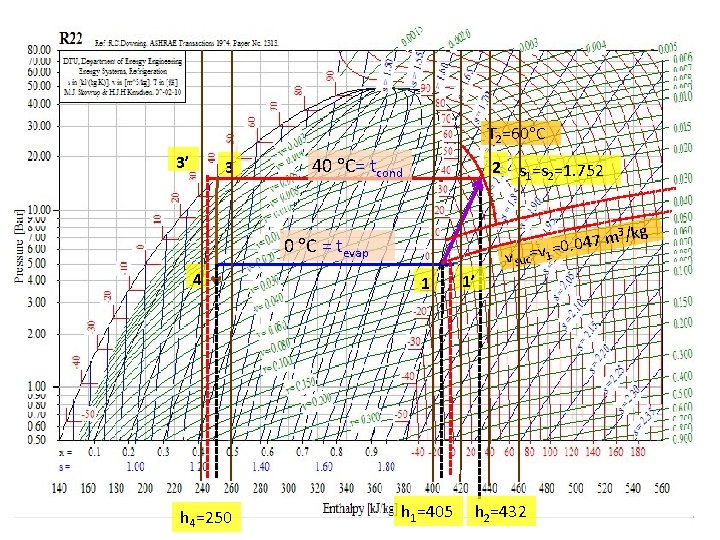
Exam 10 -2 ระบบทำความเยนแบบอดไอใช R 22 มอณหภมท Evaporator Te = 0 C และท Condenser Tc = 40 C ใหหา a) sketch เสน refrigeration cycle บน p-h diagram , b) COP ของ Carnot cycle, c) Refrigeration effect, 2 d) Work of compression, e) อณหภมของ discharge, f) COP ของ standard vapor-compression refrigeration cycle, g) ถานำ Liquid-to-suction heat exchanger มาใชเพอ superheating เพม 10 C และ subcool คาอณหภมทขาออก Condenser เปนเทาใด , h) ถาเพม อณหภม Evaporator Te เปน 10 C โดยทปรมาตร suction เทาเดม Data: VCC, R 22, te, tc , (a)p-h (b) COPcarnot (c) qe (d) wc (e)tdis (f)COP (g) t 3’ (h) w จะไดอตราการไหลเชงมวล เพมขนหรอลดลง เพราะ Method: (b) COPcarnot = T 1 / (T 2 – T 1)=6. 825 CV evaporator, energy balance, (c) qe = h 1 -h 4 CV compressor, energy balance, (d) w = (h 1 -h 2) (e) tdischarge = (f) COP = qe/wc=5. 85 CV liq-to-suc HE, energy balance, h 3 -h 3’ = h 1’-h 1 (g) tsp = 10 C, t 3’= 40 -5. 5 C (h) te w Pressure, k. Pa Assume: standard VCC; isentropic compression, isobaric, isenthalpic processes 40 C= tcond 3’ 3 4 0 C= tevap Enthalpy, k. J/kg 2 1 1’ 21

T 2=60 C 3’ 3 40 C= tcond 2 3 /kg 47 m =v 1=0. 0 0 C = tevap 4 h 4=250 s 1=s 2=1. 752 v suc 1 h 1=405 1’ h 2=432

14. 1 Analysis of Carnot refrigeration cycle T 2 high T 1, low T 2 high COP T 1 more effect on COP than T 2 T 1 Temperature, K COPR Carnot = T 1 / (T 2 – T 1) T 1 3 2 Net work 4 1 Refrigeration s 3 = s 4 s 1 = s 2 Entropy, k. J/kg K 23 23

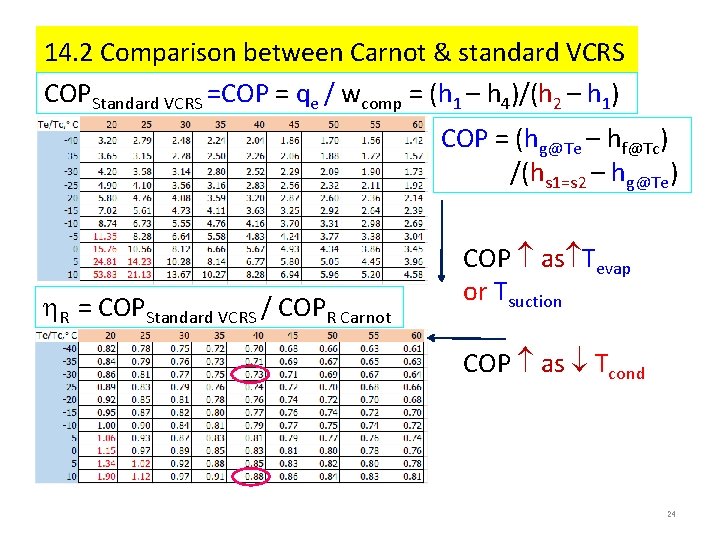
14. 2 Comparison between Carnot & standard VCRS COPStandard VCRS =COP = qe / wcomp = (h 1 – h 4)/(h 2 – h 1) COP = (hg@Te – hf@Tc) /(hs 1=s 2 – hg@Te) R = COPStandard VCRS / COPR Carnot COP as Tevap or Tsuction COP as Tcond 24

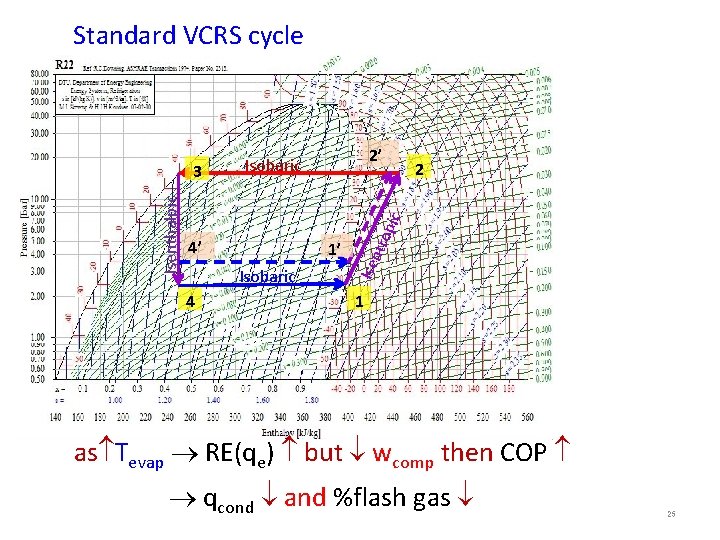
Standard VCRS cycle 4’ 1’ Isobaric 4 tro p ic 2 Isenthalpic 3 2’ Isobaric 1 as Tevap RE(qe) but wcomp then COP qcond and %flash gas 25

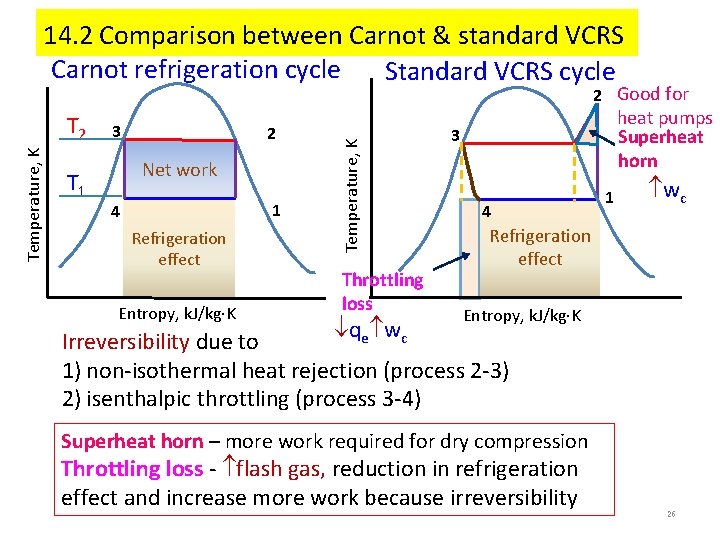
14. 2 Comparison between Carnot & standard VCRS Carnot refrigeration cycle Standard VCRS cycle Temperature, K T 2 T 1 3 2 Net work 1 4 Refrigeration effect Entropy, k. J/kg K Temperature, K 2 Good for Throttling loss heat pumps Superheat horn 3 4 1 wc Refrigeration effect Entropy, k. J/kg K qe wc Irreversibility due to 1) non-isothermal heat rejection (process 2 -3) 2) isenthalpic throttling (process 3 -4) Superheat horn – more work required for dry compression Throttling loss - flash gas, reduction in refrigeration effect and increase more work because irreversibility 26

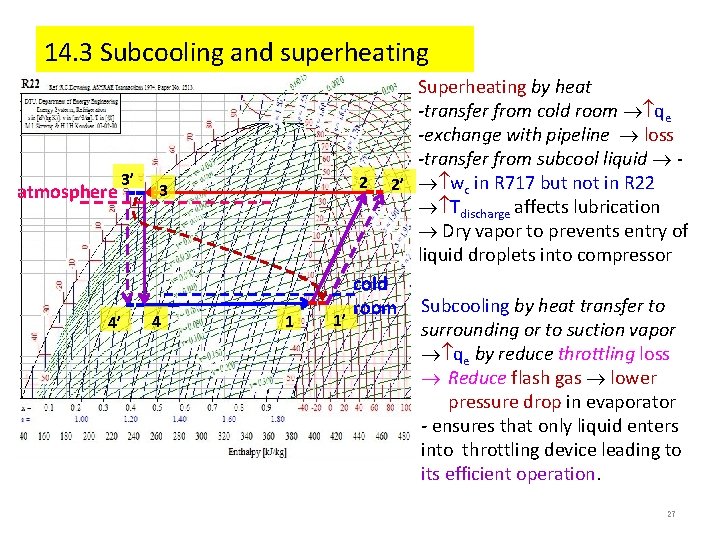
14. 3 Subcooling and superheating atmosphere 3’ 4’ 2 3 4 1 1’ Superheating by heat -transfer from cold room qe -exchange with pipeline loss -transfer from subcool liquid 2’ wc in R 717 but not in R 22 Tdischarge affects lubrication Dry vapor to prevents entry of liquid droplets into compressor cold room Subcooling by heat transfer to surrounding or to suction vapor qe by reduce throttling loss Reduce flash gas lower pressure drop in evaporator - ensures that only liquid enters into throttling device leading to its efficient operation. 27

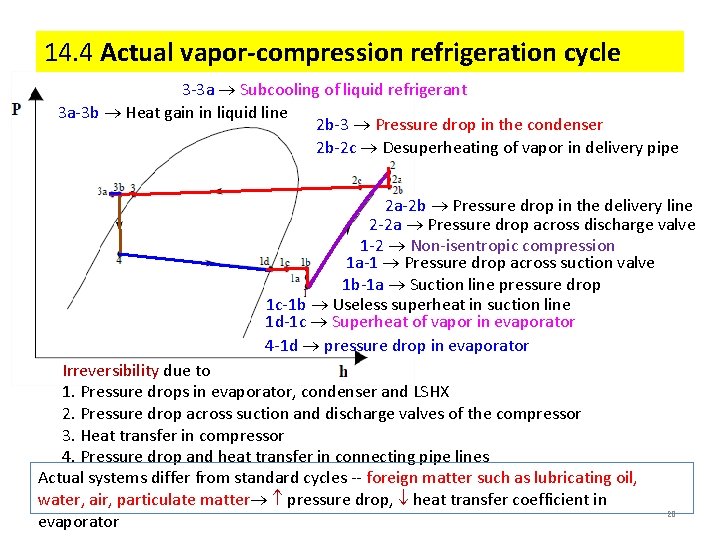
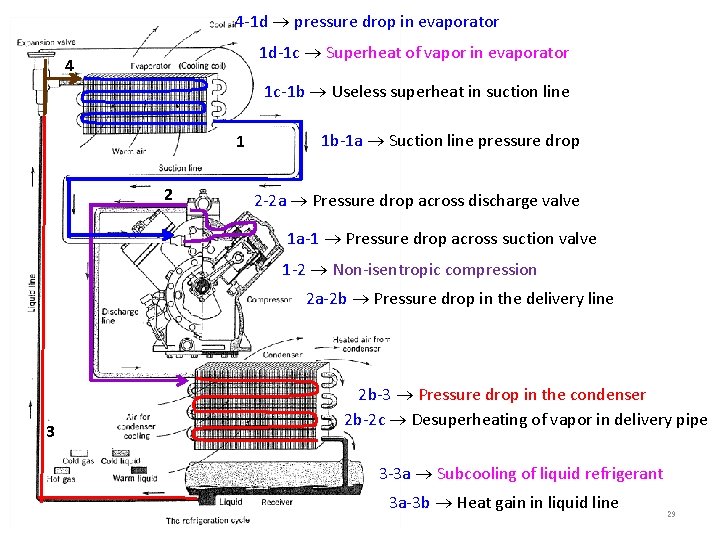
14. 4 Actual vapor-compression refrigeration cycle 3 -3 a Subcooling of liquid refrigerant 3 a-3 b Heat gain in liquid line 2 b-3 Pressure drop in the condenser 2 b-2 c Desuperheating of vapor in delivery pipe 2 a-2 b Pressure drop in the delivery line 2 -2 a Pressure drop across discharge valve 1 -2 Non-isentropic compression 1 a-1 Pressure drop across suction valve 1 b-1 a Suction line pressure drop 1 c-1 b Useless superheat in suction line 1 d-1 c Superheat of vapor in evaporator 4 -1 d pressure drop in evaporator Irreversibility due to 1. Pressure drops in evaporator, condenser and LSHX 2. Pressure drop across suction and discharge valves of the compressor 3. Heat transfer in compressor 4. Pressure drop and heat transfer in connecting pipe lines Actual systems differ from standard cycles -- foreign matter such as lubricating oil, water, air, particulate matter pressure drop, heat transfer coefficient in evaporator 28

4 -1 d pressure drop in evaporator 1 d-1 c Superheat of vapor in evaporator 4 1 c-1 b Useless superheat in suction line 1 2 1 b-1 a Suction line pressure drop 2 -2 a Pressure drop across discharge valve 1 a-1 Pressure drop across suction valve 1 -2 Non-isentropic compression 2 a-2 b Pressure drop in the delivery line 3 2 b-3 Pressure drop in the condenser 2 b-2 c Desuperheating of vapor in delivery pipe 3 -3 a Subcooling of liquid refrigerant 3 a-3 b Heat gain in liquid line 29

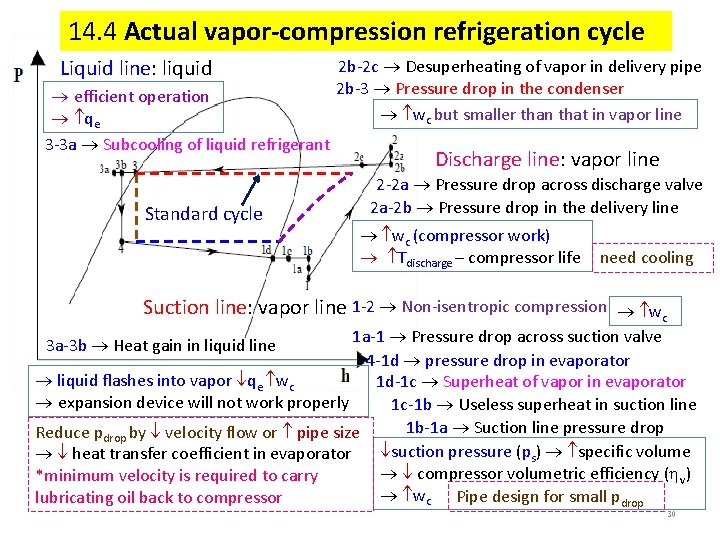
14. 4 Actual vapor-compression refrigeration cycle Liquid line: liquid efficient operation qe 3 -3 a Subcooling of liquid refrigerant Standard cycle 2 b-2 c Desuperheating of vapor in delivery pipe 2 b-3 Pressure drop in the condenser wc but smaller than that in vapor line Discharge line: vapor line 2 -2 a Pressure drop across discharge valve 2 a-2 b Pressure drop in the delivery line wc (compressor work) Tdischarge – compressor life need cooling Suction line: vapor line 1 -2 Non-isentropic compression wc 1 a-1 Pressure drop across suction valve 4 -1 d pressure drop in evaporator liquid flashes into vapor qe wc 1 d-1 c Superheat of vapor in evaporator expansion device will not work properly 1 c-1 b Useless superheat in suction line 1 b-1 a Suction line pressure drop Reduce pdrop by velocity flow or pipe size suction pressure (ps) specific volume heat transfer coefficient in evaporator compressor volumetric efficiency ( v) *minimum velocity is required to carry wc Pipe design for small pdrop lubricating oil back to compressor 3 a-3 b Heat gain in liquid line 30