Thermodynamics Lecture Series Second Law Quality of Energy




















- Slides: 31

Thermodynamics Lecture Series Second Law – Quality of Energy - Carnot Engines Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@hotmail. com http: //www 5. uitm. edu. my/faculties/fsg/drjj 1. html

Quotes • It does not matter how slowly you go, so long as you do not stop. --Confucius • To be wronged is nothing unless you continue to remember it. --Confucius

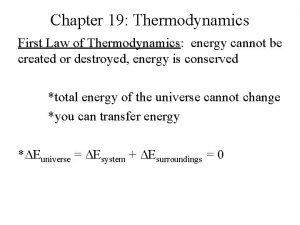
Introduction - Objectives: 1. State factors of irreversibilities in real cyclic processes. 2. Explain how each of the irreversibility factor causes energy to degrade. 3. State what is the purpose to introduce a dream engine 4. Describe and explain the processes in a Carnot engine.

Introduction - Objectives: 5. Apply the first law in each of the process of the Carnot engine. 6. Sketch a pressure-volume diagram representing a Carnot cycle and label all the energy interaction for a steam power plant. 7. Sketch a pressure-volume diagram representing a reversed Carnot cycle and label all the energy interaction for a refrigerator or heat pump.

Introduction - Objectives: 8. State the Carnot principles. 9. Use result of Carnot principles to obtain performances for a Carnot steam power plant, Carnot refrigerator and a Carnot heat pump. 10. Compare performances of Carnot engines to performances of real engines. 11. Solve problems related to Carnot engines.

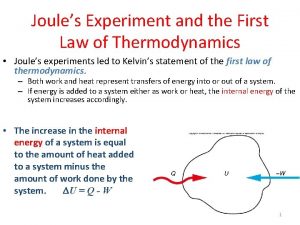
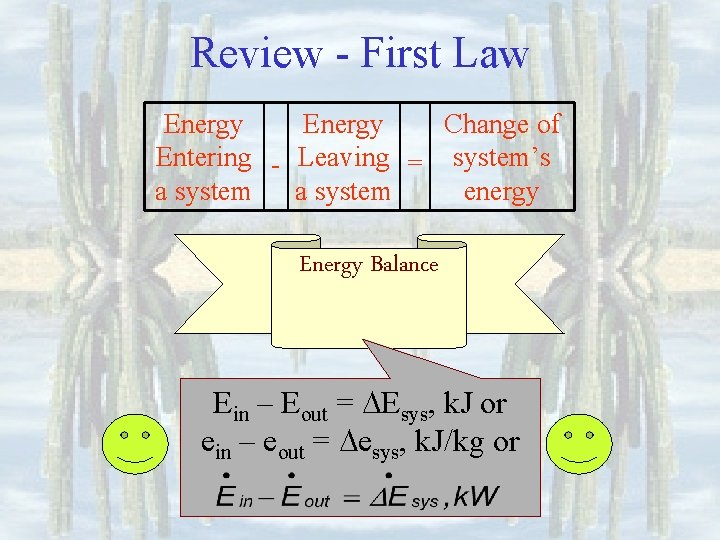
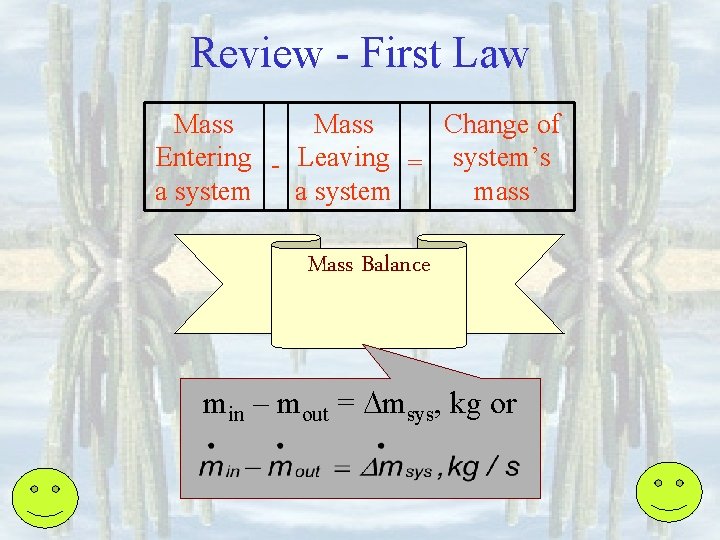
Review - First Law • All processes must obey energy conservation • Processes which do not obey energy conservation cannot happen. • Processes which do not obey mass conservation cannot happen Piston-cylinders, rigid tanks Turbines, compressors, Nozzles, heat exchangers

Review - First Law Energy Change of Entering - Leaving = system’s a system energy Energy Balance Ein – Eout = Esys, k. J or ein – eout = esys, k. J/kg or

Review - First Law Mass Change of Entering - Leaving = system’s a system mass Mass Balance min – mout = msys, kg or

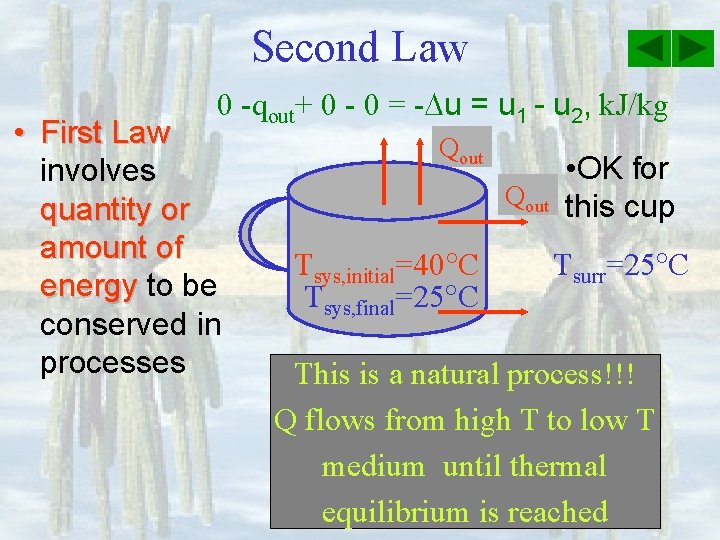
Second Law 0 -qout+ 0 - 0 = - u = u 1 - u 2, k. J/kg • First Law involves quantity or amount of energy to be conserved in processes Qout Tsys, initial=40 C Tsys, final=25 C • OK for this cup Tsurr=25 C This is a natural process!!! Q flows from high T to low T medium until thermal equilibrium is reached

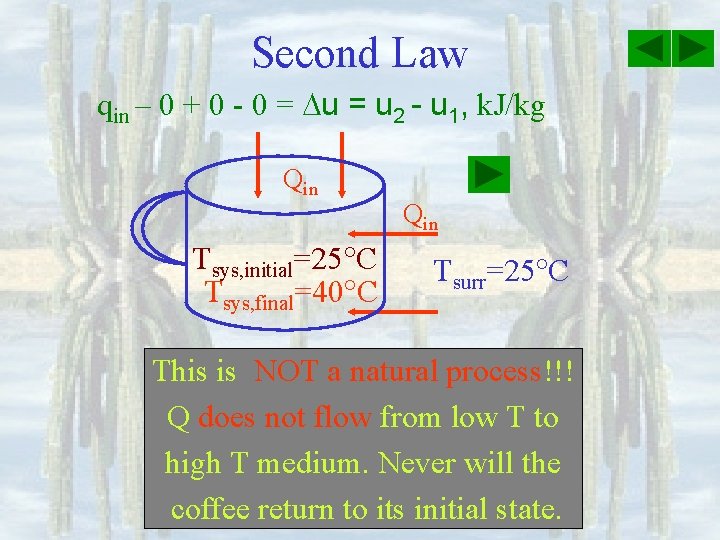
Second Law qin – 0 + 0 - 0 = u 2 - u 1, k. J/kg Qin Tsys, initial=25 C Tsys, final=40 C Qin Tsurr=25 C This is NOT a natural process!!! process Q does not flow from low T to high T medium. Never will the coffee return to its initial state.

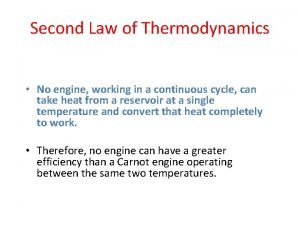
Second Law • First Law is not sufficient to determine if a process can or cannot proceed • Introduce the second law of thermodynamics – processes occur in its natural direction. Ø Heat (thermal energy) flows from high temperature medium to low temperature medium. Ø Energy has quality & quality is higher with higher temperature. More work can be done.

Second Law Considerations: • Work can be converted to heat directly & totally. • Heat cannot be converted to work directly & totally. Ø Requires a special device – heat engine.

Working fluid: Water qin - qout = out - in Second Law High T Res. , TH Furnace qin = q. H qin = net, out + qout Purpose: Produce work, Wout, out Steam Power Plant net, out = qin - qout = q. L Low T Res. , TL Water from river An Energy-Flow diagram for a SPP net, out

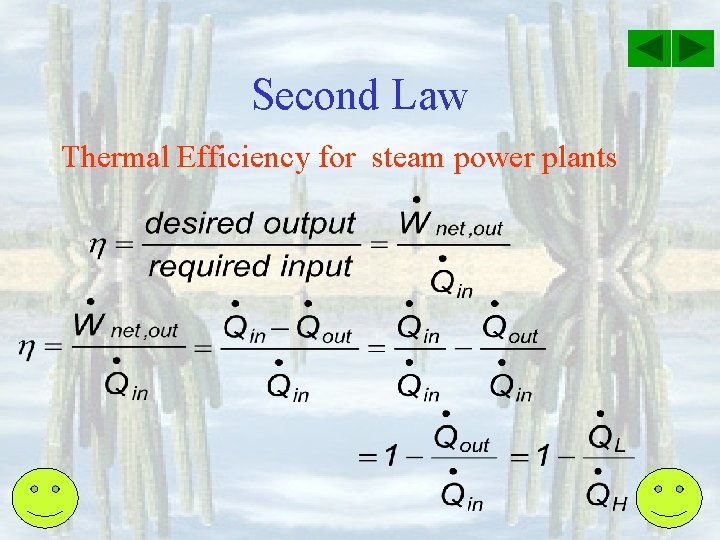
Second Law Thermal Efficiency for steam power plants

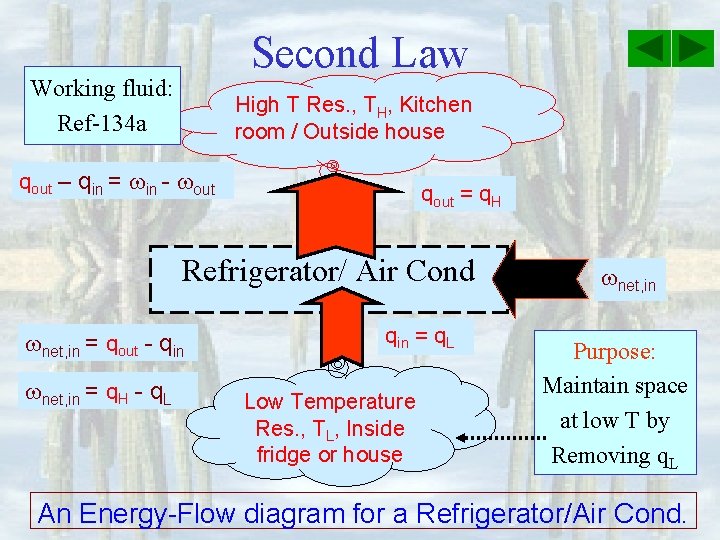
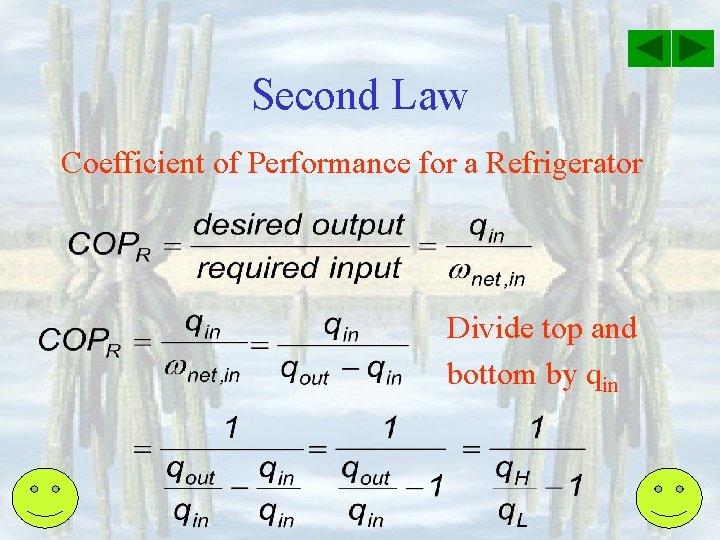
Second Law Working fluid: Ref-134 a High T Res. , TH, Kitchen room / Outside house qout – qin = in - out qout = q. H Refrigerator/ Air Cond net, in = qout - qin net, in = q. H - q. L qin = q. L Low Temperature Res. , TL, Inside fridge or house net, in Purpose: Maintain space at low T by Removing q. L An Energy-Flow diagram for a Refrigerator/Air Cond.

Second Law Coefficient of Performance for a Refrigerator Divide top and bottom by qin

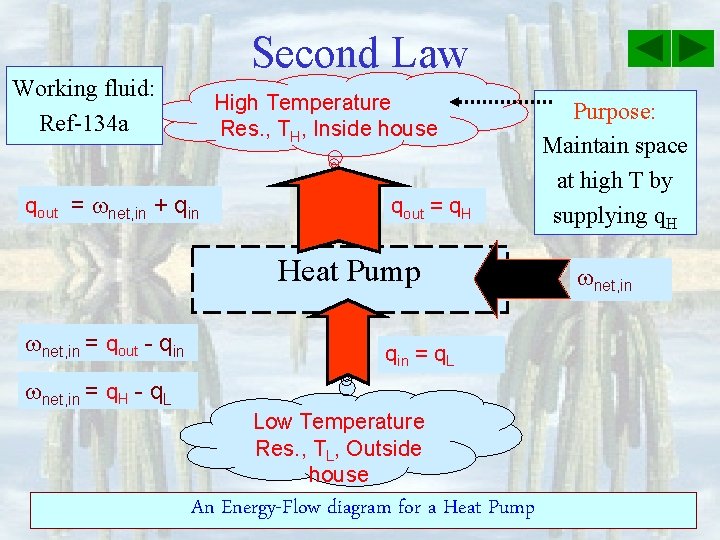
Second Law Working fluid: Ref-134 a High Temperature Res. , TH, Inside house qout = net, in + qin qout = q. H Heat Pump net, in = qout - qin = q. L net, in = q. H - q. L Low Temperature Res. , TL, Outside house An Energy-Flow diagram for a Heat Pump Purpose: Maintain space at high T by supplying q. H net, in

Second Law Coefficient of Performance for a Heat Pump

Second Law – Energy Degrade What is the maximum performance of real engines if it can never achieve 100%? ? • Processes in real devices are irreversible Factors of irreversibilities • less heat can be converted to work – Friction between 2 moving surfaces – Processes happen too fast – Non-isothermal heat transfer

Second Law – Energy Degrade What is the maximum performance of real engines if it can never achieve 100%? ? Factors of irreversibilities • Friction between 2 moving surfaces – Heat generated during compression causes wall of cylinder to increase – Work supplied by surrounding is lost to warming of cylinder walls. – At end of cycle, if piston can be returned to original state, surrounding cannot be returned to original state ( work in not equal to work out).

Second Law – Energy Degrade What is the maximum performance of real engines if it can never achieve 100%? ? Factors of irreversibilities • Processes happen too fast – Fast compression causes pressure near piston to be higher than pressure at bottom due to molecules near piston not enough time to react. – Hence more work is required to compress system. (work in bigger than work out) System

Second Law – Energy Degrade What is the maximum performance of real engines if it can never achieve 100%? ? Factors of irreversibilities • Heat transfer at finite temperature difference – Surrounding loses Q during warming of coke. – Surrounding loses W but supply Q during return process. – After cycle, surrounding not returned to original state since the work supplied by surrounding was not returned to the surrounding.

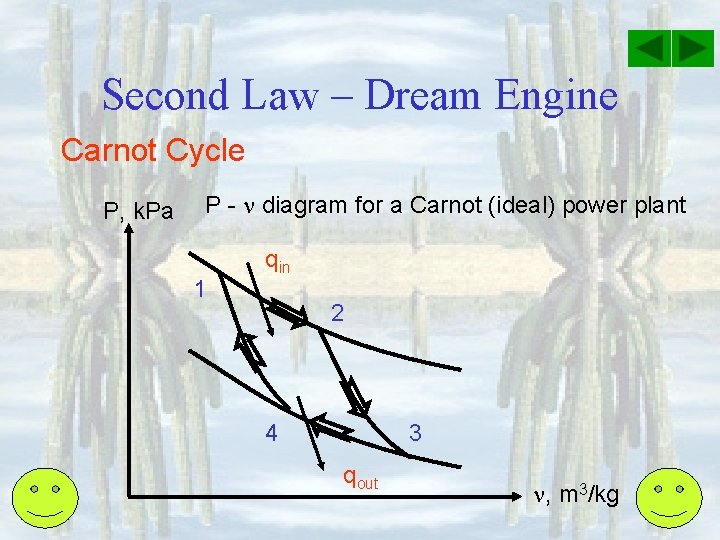
Second Law – Dream Engine Carnot Cycle-All processes in cycle is completely reversible. Hence performance is the highest. • Isothermal expansion v. Slow adding of Q resulting in work done by system (system expand) v. Qin – Wout = U = 0. So, Qin = Wout. Pressure drops.

Second Law – Dream Engine Carnot Cycle • Adiabatic expansion v 0 – Wout = U. Final U smaller than initial U. v. T & P drops.

Second Law – Dream Engine Carnot Cycle • Isothermal compression v. Work done on the system v. Slow rejection of Q v- Qout + Win = U = 0. So, Qout = Win. v. Pressure increases.

Second Law – Dream Engine Carnot Cycle • Adiabatic compression v 0 + Win = U. Final U higher than initial U. v. T & P increases.

Second Law – Dream Engine Carnot Cycle P, k. Pa P - diagram for a Carnot (ideal) power plant 1 qin 2 4 3 qout , m 3/kg

Second Law – Dream Engine Reverse Carnot Cycle P, k. Pa P - diagram for a Carnot (ideal) refrigerator 1 qout 4 2 3 qin , m 3/kg

Second Law – Dream Engine Carnot Principles • For heat engines in contact with the same hot and cold reservoir v. All reversible engines have the same performance. v. Real engines will have lower performance than the ideal engines.

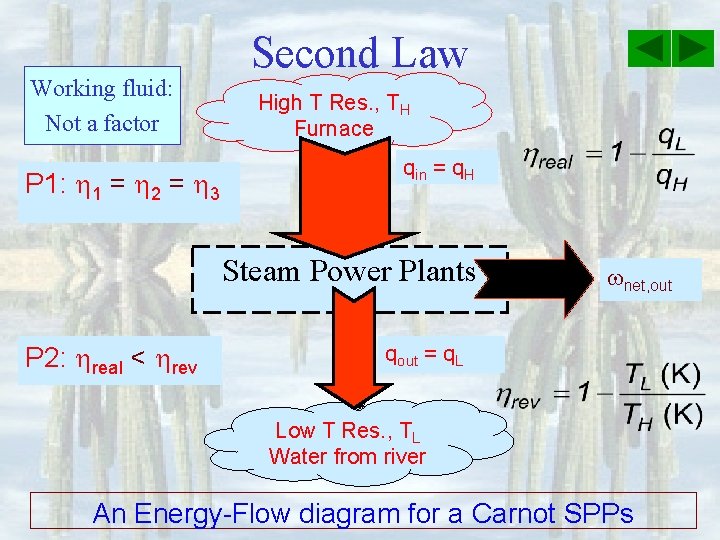
Working fluid: Not a factor P 1: 1 = 2 = 3 Second Law High T Res. , TH Furnace qin = q. H Steam Power Plants P 2: real < rev net, out qout = q. L Low T Res. , TL Water from river An Energy-Flow diagram for a Carnot SPPs

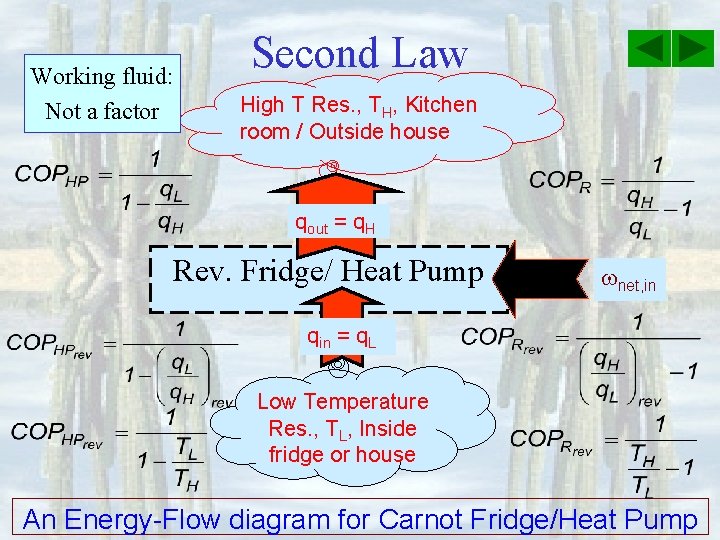
Working fluid: Not a factor Second Law High T Res. , TH, Kitchen room / Outside house qout = q. H Rev. Fridge/ Heat Pump net, in qin = q. L Low Temperature Res. , TL, Inside fridge or house An Energy-Flow diagram for Carnot Fridge/Heat Pump
 Kelvin statement
Kelvin statement State second law of thermodynamics
State second law of thermodynamics What is second law of thermodynamics
What is second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law of motion
Newton's first law of motion 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad 27 miles per gallon into kilometers per liter
27 miles per gallon into kilometers per liter Geology lecture series
Geology lecture series Dcac lecture series
Dcac lecture series First law of thermodynamics for open system
First law of thermodynamics for open system Thermodynamic
Thermodynamic Newtons third law of thermodynamics
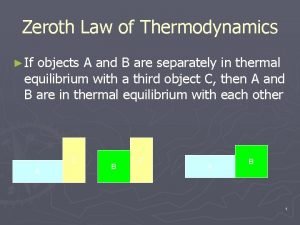
Newtons third law of thermodynamics Zeroth law of thermodynamics statement
Zeroth law of thermodynamics statement Isobaric process formula
Isobaric process formula 1th law of thermodynamics
1th law of thermodynamics Joules experiment
Joules experiment 1st law of thermodynamics
1st law of thermodynamics First law of thermodynamics for steady state flow process
First law of thermodynamics for steady state flow process Law of thermodynamics
Law of thermodynamics Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Third law of thermodynamics is depend on
Third law of thermodynamics is depend on Dq=cdt
Dq=cdt First law of thermodynamics sign convention
First law of thermodynamics sign convention 1st law of thermodynamics
1st law of thermodynamics 1st law of thermodynamics
1st law of thermodynamics First law of thermodynamics control mass
First law of thermodynamics control mass Zeroth law of thermodynamics
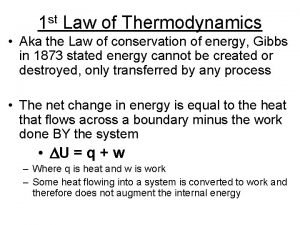
Zeroth law of thermodynamics First law of thermodynamics
First law of thermodynamics