Thermodynamics 1 st law of thermodynamics Energy may







- Slides: 23

Thermodynamics

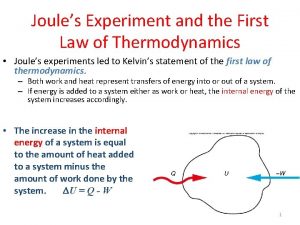
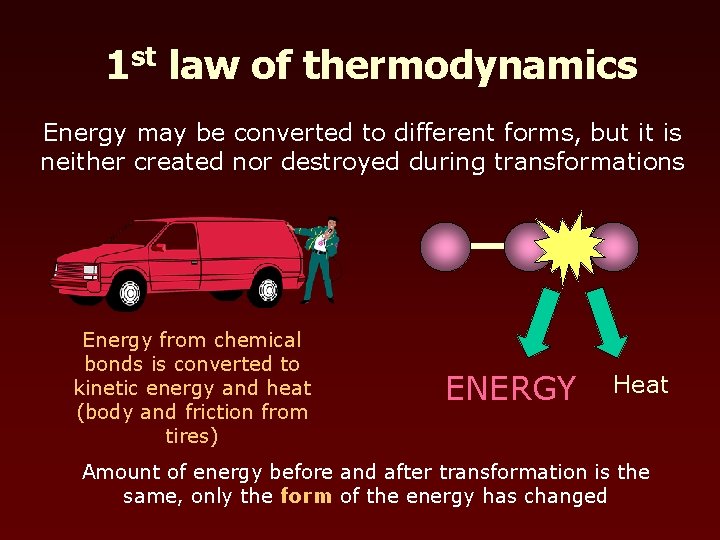
1 st law of thermodynamics Energy may be converted to different forms, but it is neither created nor destroyed during transformations Energy from chemical bonds is converted to kinetic energy and heat (body and friction from tires) ENERGY Heat Amount of energy before and after transformation is the same, only the form of the energy has changed

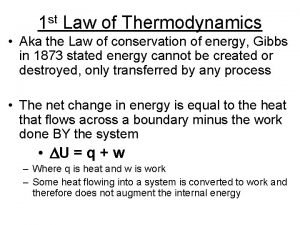
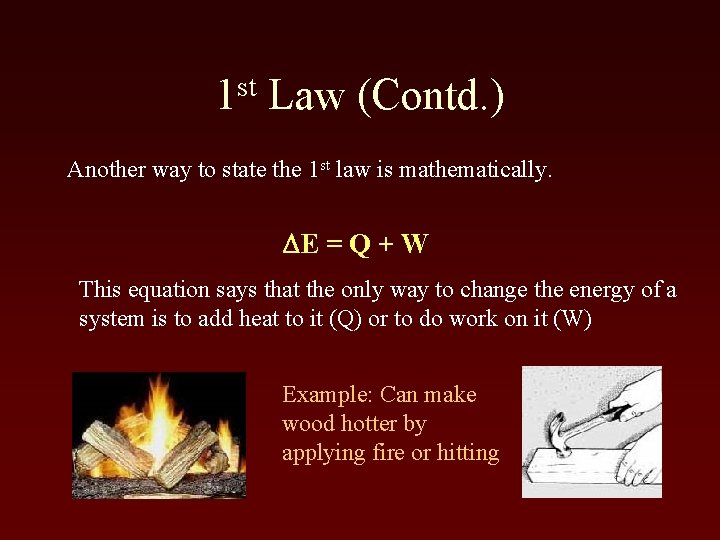
st 1 Law (Contd. ) Another way to state the 1 st law is mathematically. DE = Q + W This equation says that the only way to change the energy of a system is to add heat to it (Q) or to do work on it (W) Example: Can make wood hotter by applying fire or hitting

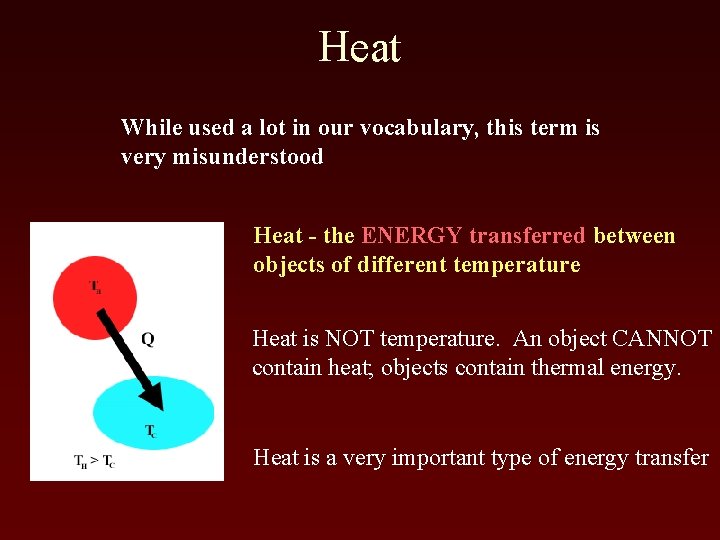
Heat While used a lot in our vocabulary, this term is very misunderstood Heat - the ENERGY transferred between objects of different temperature Heat is NOT temperature. An object CANNOT contain heat; objects contain thermal energy. Heat is a very important type of energy transfer

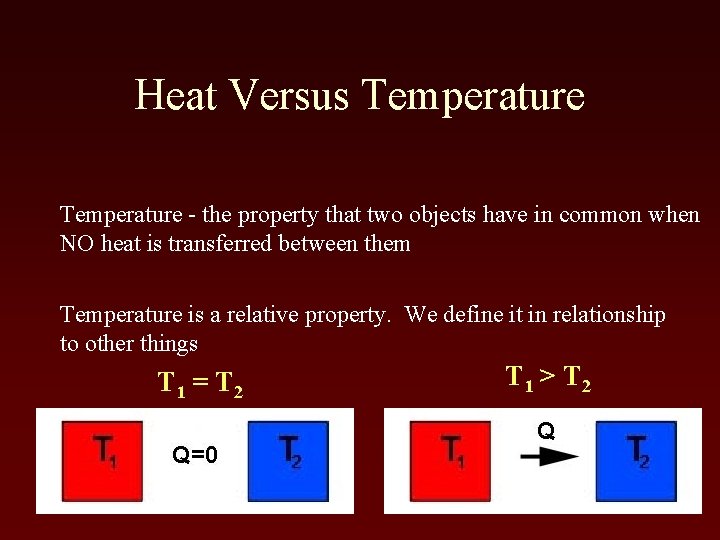
Heat Versus Temperature - the property that two objects have in common when NO heat is transferred between them Temperature is a relative property. We define it in relationship to other things T 1 = T 2 T 1 > T 2

Heat Flow Heat can flow via one of three methods 1. Conduction - energy transfer by next-nearest molecule interaction 2. Convection - energy transfer by mixing; can be natural or forced (fan, stirring, etc. ) 3. Radiation - energy transfer by electromagnetic radiation

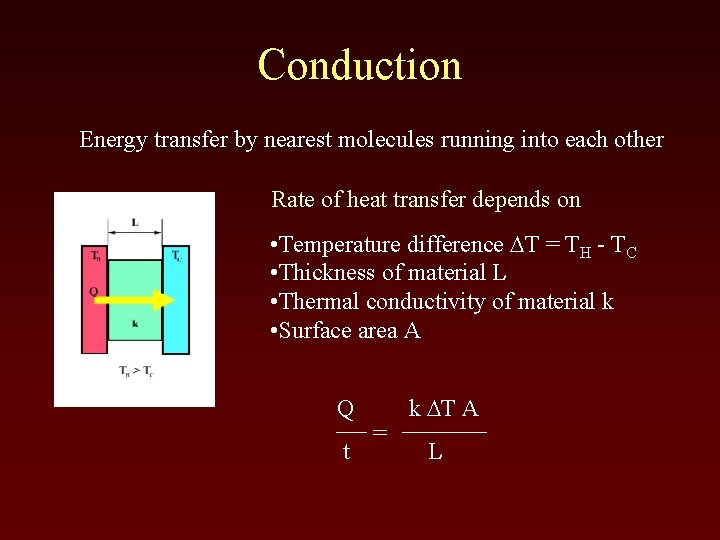
Conduction Energy transfer by nearest molecules running into each other Rate of heat transfer depends on • Temperature difference DT = TH - TC • Thickness of material L • Thermal conductivity of material k • Surface area A Q t = k DT A L

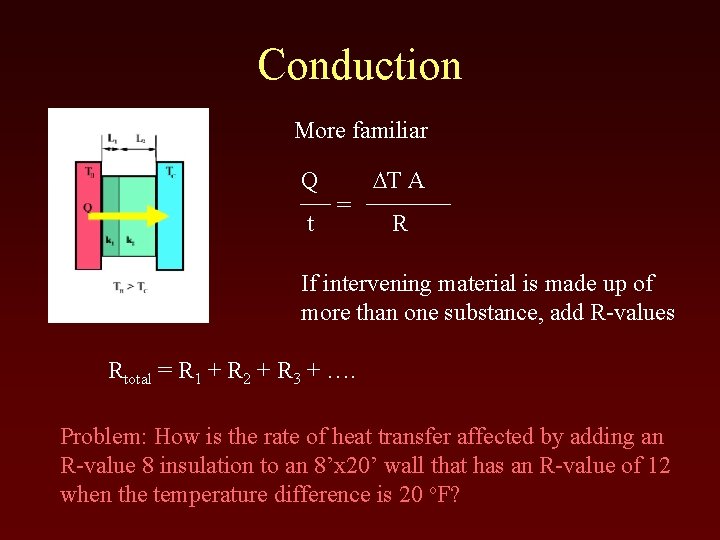
Conduction More familiar Q t = DT A R If intervening material is made up of more than one substance, add R-values Rtotal = R 1 + R 2 + R 3 + …. Problem: How is the rate of heat transfer affected by adding an R-value 8 insulation to an 8’x 20’ wall that has an R-value of 12 when the temperature difference is 20 o. F?

Convection Heat transfer via mixing; requires some type of fluid (gas, liquid) Things can naturally convect, especially when density changes and more buoyant materials will rise Forced convection requires energy input

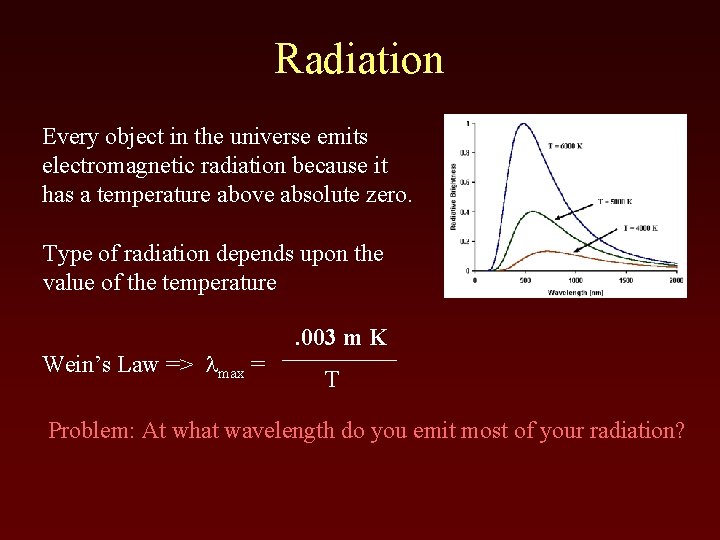
Radiation Every object in the universe emits electromagnetic radiation because it has a temperature above absolute zero. Type of radiation depends upon the value of the temperature Wein’s Law => lmax = . 003 m K T Problem: At what wavelength do you emit most of your radiation?

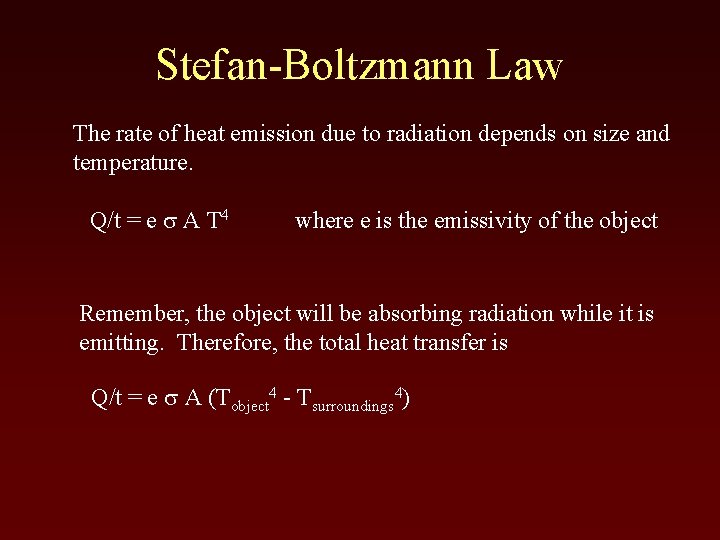
Stefan-Boltzmann Law The rate of heat emission due to radiation depends on size and temperature. Q/t = e s A T 4 where e is the emissivity of the object Remember, the object will be absorbing radiation while it is emitting. Therefore, the total heat transfer is Q/t = e s A (Tobject 4 - Tsurroundings 4)

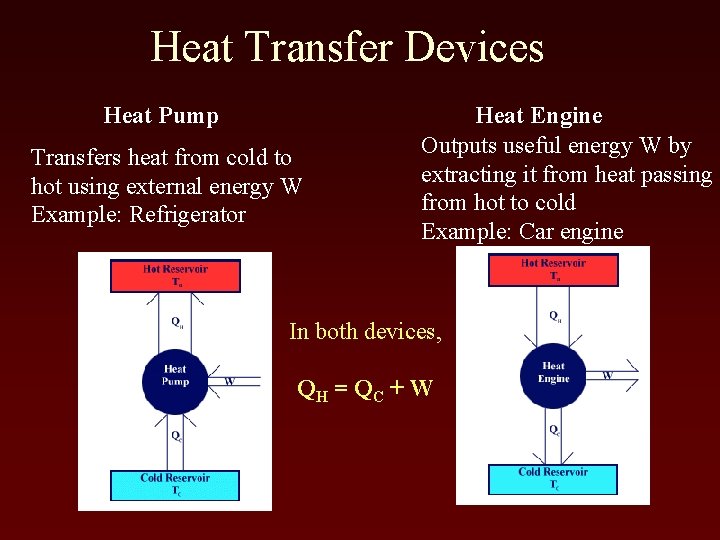
Heat Transfer Devices Heat Pump Transfers heat from cold to hot using external energy W Example: Refrigerator Heat Engine Outputs useful energy W by extracting it from heat passing from hot to cold Example: Car engine In both devices, QH = Q C + W

2 nd law of thermodynamics If energy is never created or destroyed, why can’t we keep reusing the same energy source forever? ANSWER: Although energy isn’t destroyed, in every energy transfer, some of it will change to a non-usable form This is a consequence of the 2 nd law of thermodynamics “In a closed system, the total entropy either increases or stays the same”

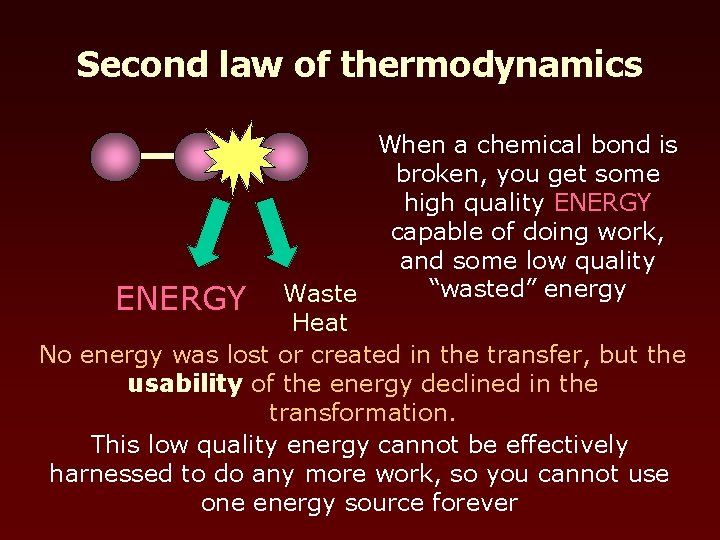
Second law of thermodynamics When a chemical bond is broken, you get some high quality ENERGY capable of doing work, and some low quality “wasted” energy ENERGY Waste Heat No energy was lost or created in the transfer, but the usability of the energy declined in the transformation. This low quality energy cannot be effectively harnessed to do any more work, so you cannot use one energy source forever

Second law of thermodynamics Example: powering your car Breaking chemical bonds in gas during combustion yields high quality energy which produces kinetic energy to move car Also produces waste energy as heat with little ability to do work

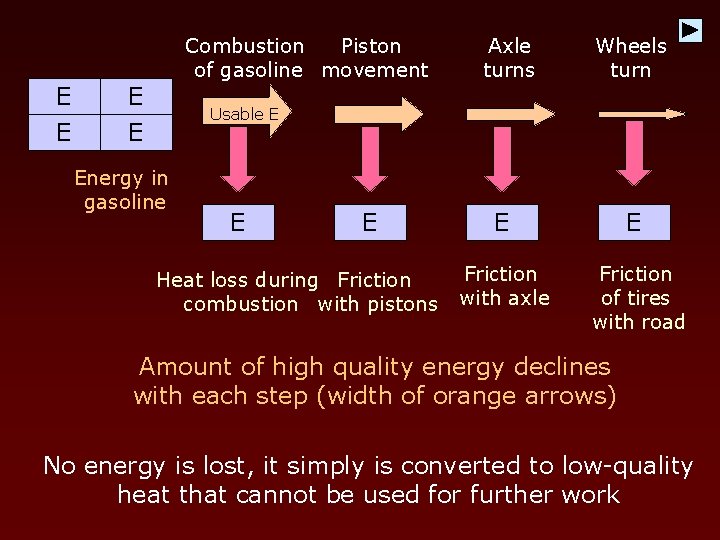
Combustion Piston of gasoline movement E E Axle turns Wheels turn Usable E Energy in gasoline E E Heat loss during Friction combustion with pistons E E Friction with axle Friction of tires with road Amount of high quality energy declines with each step (width of orange arrows) No energy is lost, it simply is converted to low-quality heat that cannot be used for further work

Efficiency A measure of how well energy is converted Efficiency = useful energy out total energy input Examples Internal combustion engine car is about 10% efficient Electric car is about 20% efficient Incandescent light bulb is about 1% efficient

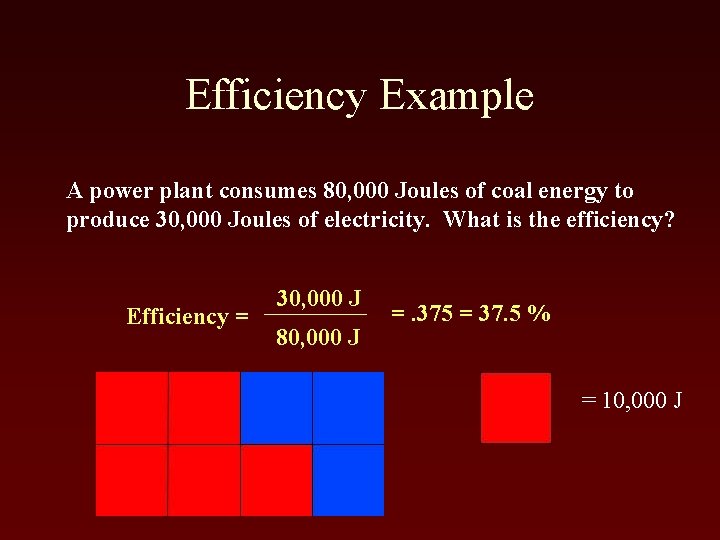
Efficiency Example A power plant consumes 80, 000 Joules of coal energy to produce 30, 000 Joules of electricity. What is the efficiency? Efficiency = 30, 000 J 80, 000 J =. 375 = 37. 5 % = 10, 000 J

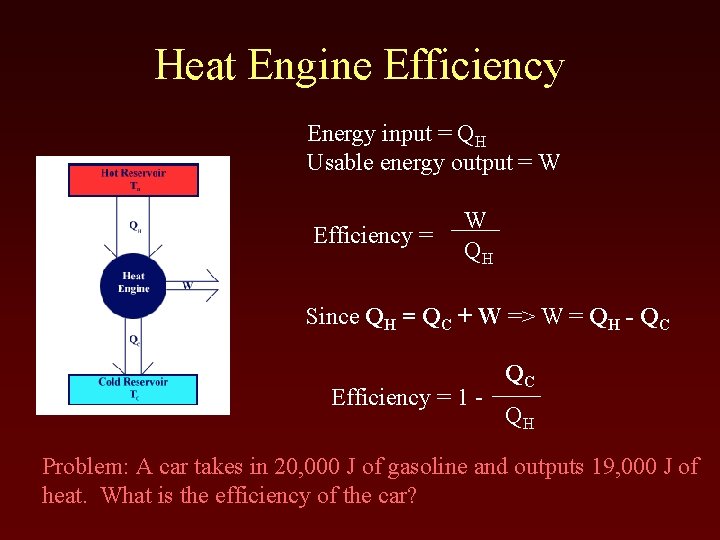
Heat Engine Efficiency Energy input = QH Usable energy output = W Efficiency = W QH Since QH = QC + W => W = QH - QC Efficiency = 1 - QC QH Problem: A car takes in 20, 000 J of gasoline and outputs 19, 000 J of heat. What is the efficiency of the car?

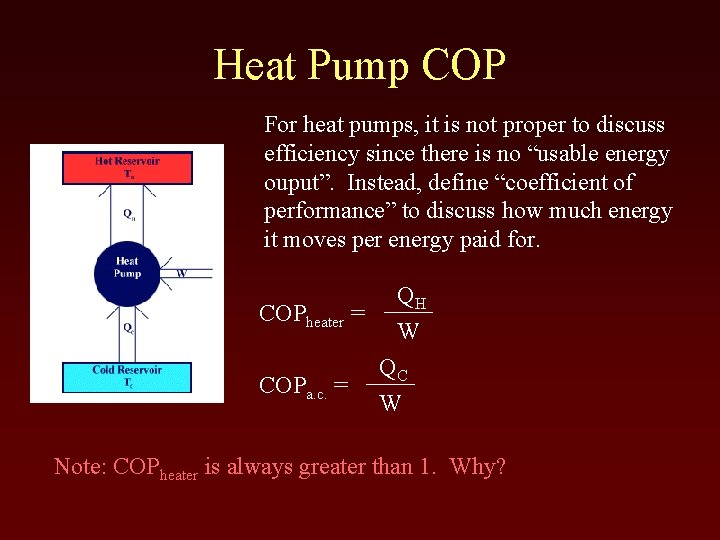
Heat Pump COP For heat pumps, it is not proper to discuss efficiency since there is no “usable energy ouput”. Instead, define “coefficient of performance” to discuss how much energy it moves per energy paid for. COPheater = COPa. c. = QH W QC W Note: COPheater is always greater than 1. Why?

Maximum Efficiency Unfortunately, the 2 nd law of thermodynamics limits the maximum efficiency that a device can have. No device will ever be 100% efficient. For a heat engine, the limit is given by Maximum efficiency = 1 - TC TH where TC is the temperature of the cold reservoir and TH is the temperature of the hot reservoir in the Kelvin temperature scale

Maximum Efficiency Example An inventor proposes a heat engine that will produce electricity by extracting heat from ocean surface water at 20 o. C (293 K) and dumping the waste heat to the deep ocean at 5 o. C (278 K). What is the maximum efficiency? Maximum efficiency = 1 - 278 K = 1 -. 95 =. 05 293 K At most, this device will be 5% efficient. In reality, it will probably only be about half of this, or 2 -3% efficient.

Recapping 1 st LAW: Energy is neither created nor destroyed, only transformed 2 nd LAW: Energy is transformed from high quality to low quality RESULT: Low quality heat cannot do substantial work, requiring a new source of high quality energy
 Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law of motion
Newton's first law of motion Boyles law
Boyles law Avogadro's law constants
Avogadro's law constants Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis First law of thermodynamics in open system
First law of thermodynamics in open system Oth law of thermodynamics
Oth law of thermodynamics Newtons third law of thermodynamics
Newtons third law of thermodynamics Zeroth law of thermodynamics examples
Zeroth law of thermodynamics examples Isobaric process formula
Isobaric process formula Brayton cycle process
Brayton cycle process Isentropic compressor
Isentropic compressor Zeroth law of thermodynamics
Zeroth law of thermodynamics Joule's first law
Joule's first law Isothermal process in thermodynamics
Isothermal process in thermodynamics First law applied to flow process
First law applied to flow process Second law of thermodynamics
Second law of thermodynamics What are the laws of thermodynamics
What are the laws of thermodynamics Second law of thermodynamics
Second law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Third law of thermodynamics is depend on
Third law of thermodynamics is depend on Dq=cdt
Dq=cdt