Thermodynamics Lecture Series Second Law Quality of EnergyPart



























- Slides: 57

Thermodynamics Lecture Series Second Law – Quality of Energy-Part 1 Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@hotmail. com http: //www 5. uitm. edu. my/faculties/fsg/drjj 1. html

Quotes • It does not matter how slowly you go, so long as you do not stop. --Confucius • To be wronged is nothing unless you continue to remember it. --Confucius


Symbols • • Q q W V E

Introduction - Objectives: 1. Explain the need for the second law of thermodynamics on real processes. 2. State the general and the specific statements of the second law of thermodynamics. 3. State the meaning of reservoirs and working fluids. 4. List down the characteristics of heat engines.

Introduction - Objectives: 5. State the difference between thermodynamic heat engines and mechanical heat engines. 6. Sketch an energy-flow diagram indicating the flow of energy and label all the energies and all the reservoirs for a steam power plant. 7. Sketch a schematic diagram for a steam power plant and label all the energies, flow of energies and all the reservoirs.

Introduction - Objectives: 8. State the desired output and required input for a steam power plant. 9. State the meaning of engines’ performance and obtain the performance of a steam power plant in terms of the heat exchange. 10. State the Kelvin-Planck statement on steam power plant.

Introduction - Objectives: 11. Sketch an energy-flow diagram indicating the flow of energy and label all the energies and all the reservoirs for a refrigerator. 12. Sketch a schematic diagram for a refrigerator and label all the energies, flow of energies and all the reservoirs. 13. State the desired output and required input for a refrigerator.

Introduction - Objectives: 14. Obtain the performance of a refrigerator in terms of the heat exchange. 15. Sketch an energy-flow diagram indicating the flow of energy and label all the energies and all the reservoirs for a heat pump. 16. Sketch a schematic diagram for a a heat pump and label all the energies, flow of energies and all the reservoirs.

Introduction - Objectives: 17. State the desired output and required input for a a heat pump. 18. Obtain the performance of a a heat pump in terms of the heat exchange. 19. State the Clausius statement for refrigerators and heat pumps. 20. Solve problems related to steam power plants, refrigerators and heat pumps.

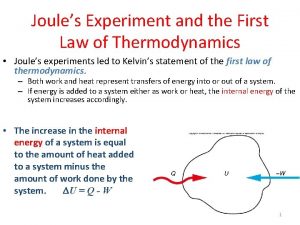
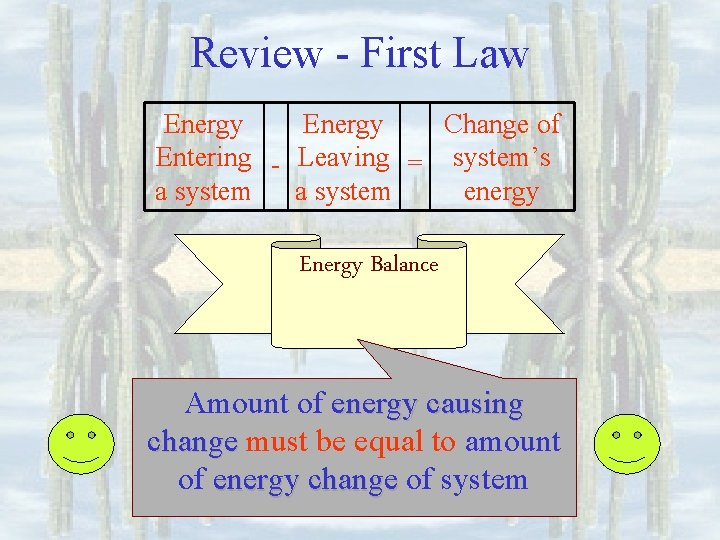
Review - First Law • All processes must obey energy conservation • Processes which do not obey energy conservation cannot happen. • Processes which do not obey mass conservation cannot happen Piston-cylinders, rigid tanks Turbines, compressors, Nozzles, heat exchangers

Review - First Law How to relate changes to the cause Mass in Qout Properties will change indicating change of state System E 1, P 1, T 1, V 1 To E 2, P 2, T 2, V 2 Dynamic Energies as causes (agents) of change Win Wout Mass out

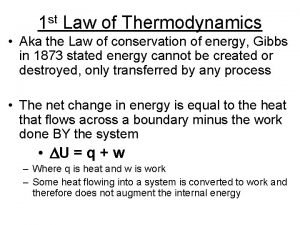
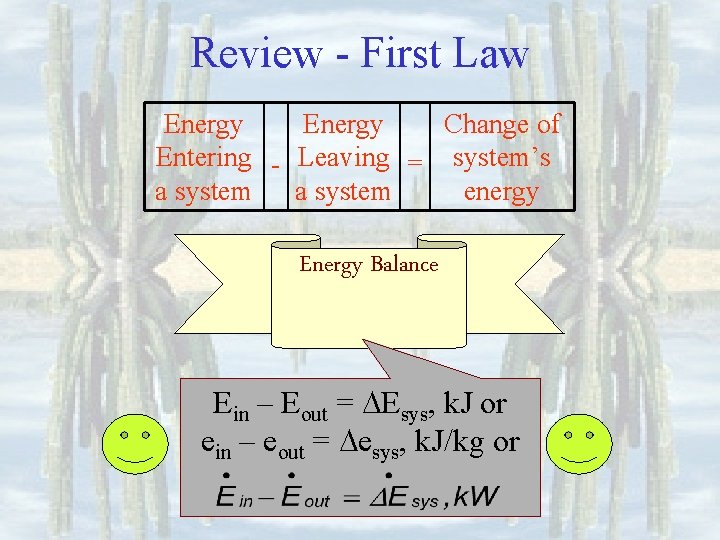
Review - First Law Energy Change of Entering - Leaving = system’s a system energy Energy Balance Amount of energy causing change must be equal to amount of energy change of system

Review - First Law Energy Change of Entering - Leaving = system’s a system energy Energy Balance Ein – Eout = Esys, k. J or ein – eout = esys, k. J/kg or

Review - First Law Mass Change of Entering - Leaving = system’s a system mass Mass Balance min – mout = msys, kg or

Review - First Law Energy Balance – Control Volume Steady-Flow Steady-flow is a flow where all properties within boundary of the system remains constant with time Esys= 0, k. J; esys= 0 , k. J/kg, Vsys= 0, m 3; msys= 0 or min = mout , kg

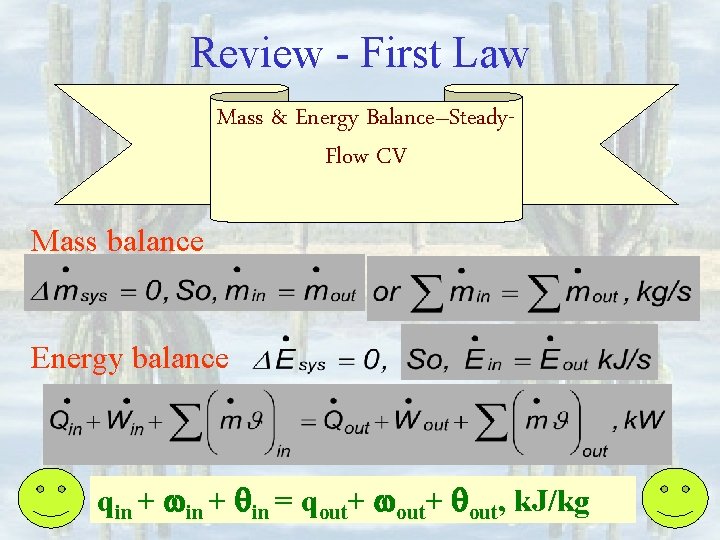
Review - First Law Mass & Energy Balance–Steady. Flow CV Mass balance Energy balance qin + win + qin = qout+ wout+ qout, k. J/kg

Review - First Law Mass & Energy Balance–Steady. Flow: Single Stream Mass balance Energy balance qin – qout+ in – out = qout – qin, k. J/kg = hout - hin + keout– kein + peout - pein, k. J/kg

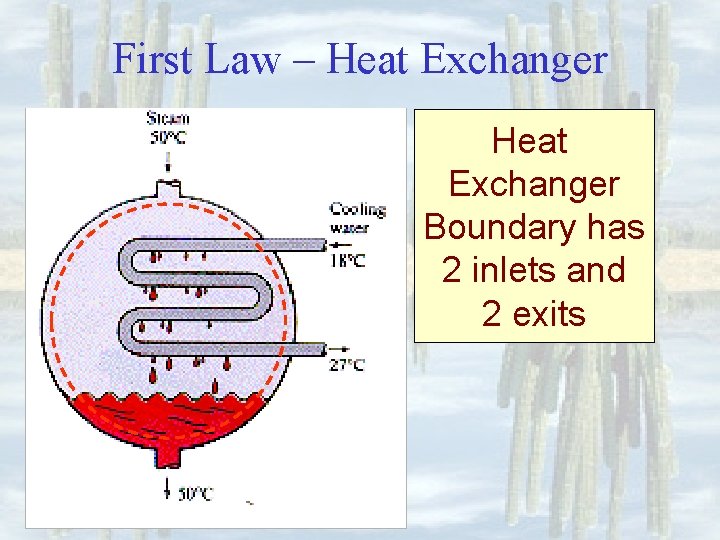
First Law – Heat Exchanger Boundary has 2 inlets and 2 exits

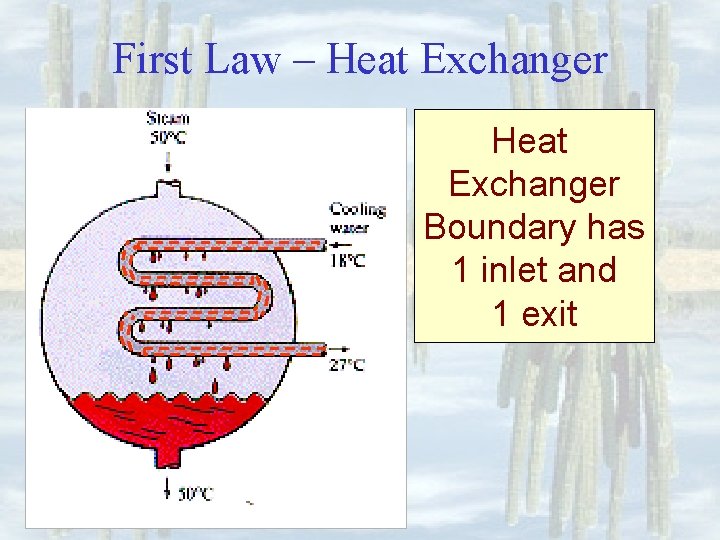
First Law – Heat Exchanger Boundary has 1 inlet and 1 exit

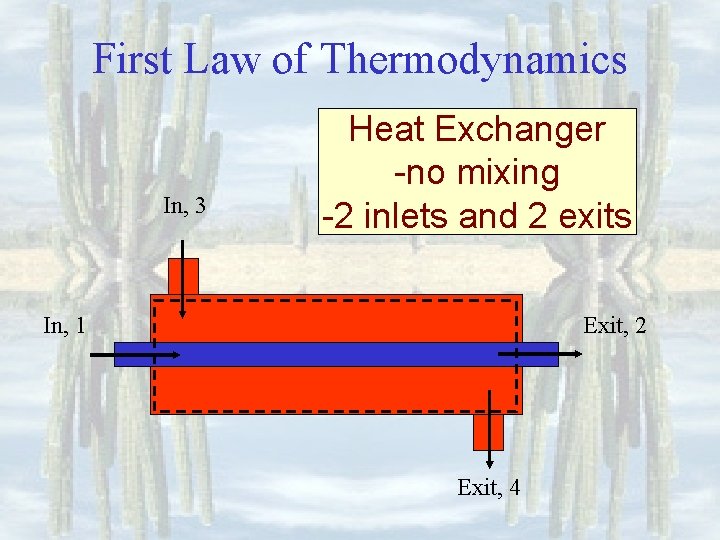
First Law of Thermodynamics In, 3 Heat Exchanger -no mixing -2 inlets and 2 exits In, 1 Exit, 2 Exit, 4

First Law of Thermodynamics In, 3 Heat Exchanger -no mixing -1 inlet and 1 exit In, 1 Exit, 2 Exit, 4

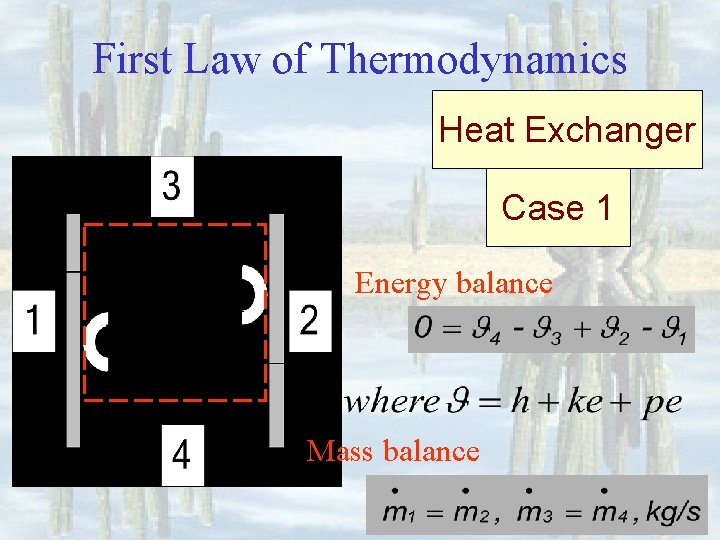
First Law of Thermodynamics Heat Exchanger Case 1 Energy balance Mass balance

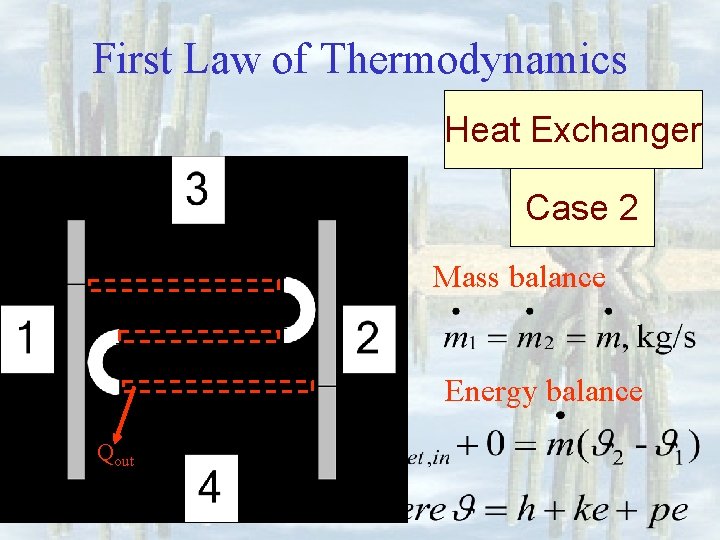
First Law of Thermodynamics Heat Exchanger Case 2 Mass balance Energy balance Qout

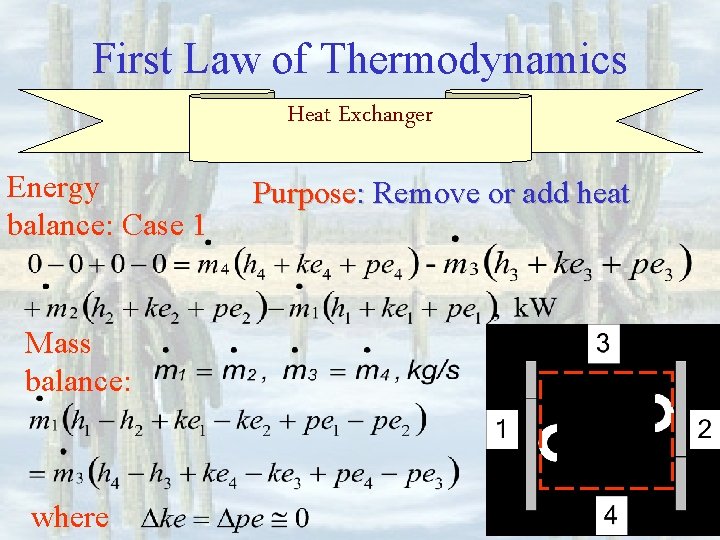
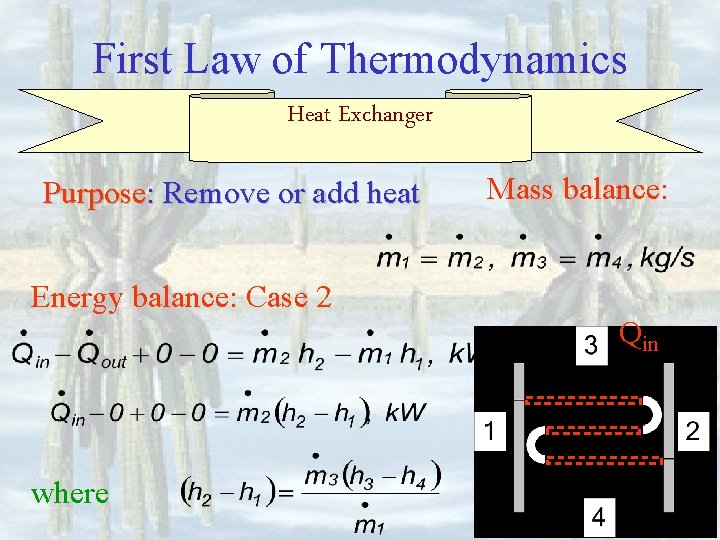
First Law of Thermodynamics Heat Exchanger Energy balance: Case 1 Mass balance: where Purpose: Remove or add heat

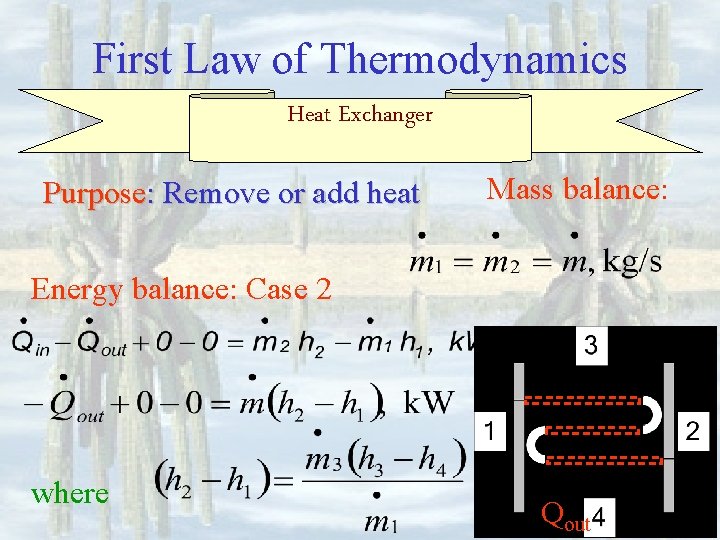
First Law of Thermodynamics Heat Exchanger Purpose: Remove or add heat Mass balance: Energy balance: Case 2 where Qout

First Law of Thermodynamics Heat Exchanger Purpose: Remove or add heat Mass balance: Energy balance: Case 2 Qin where

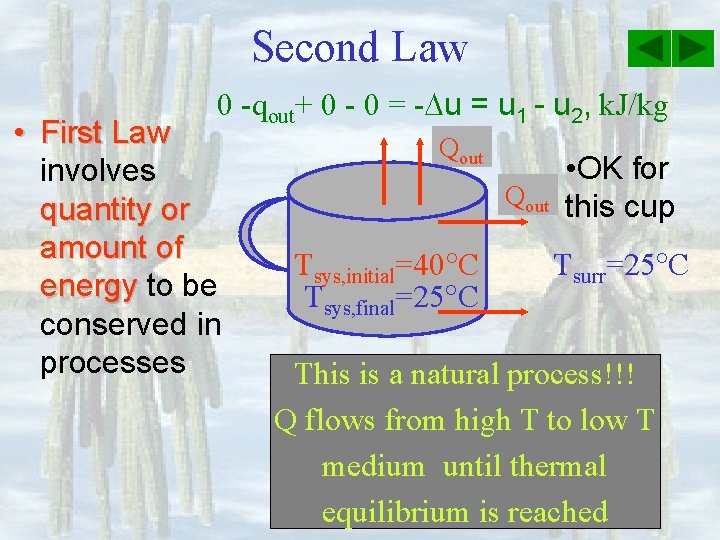
Second Law 0 -qout+ 0 - 0 = - u = u 1 - u 2, k. J/kg • First Law involves quantity or amount of energy to be conserved in processes Qout Tsys, initial=40 C Tsys, final=25 C • OK for this cup Tsurr=25 C This is a natural process!!! Q flows from high T to low T medium until thermal equilibrium is reached

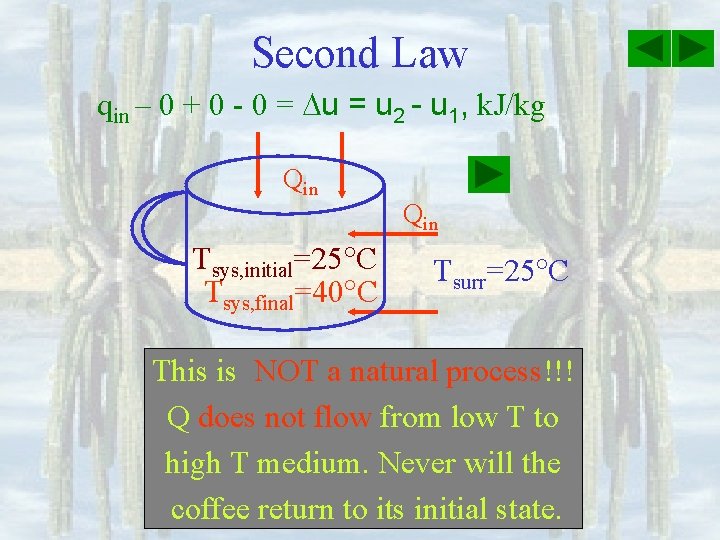
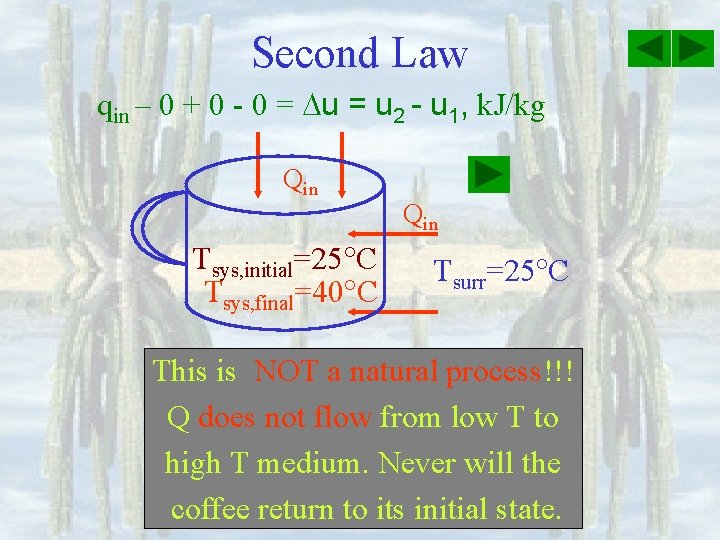
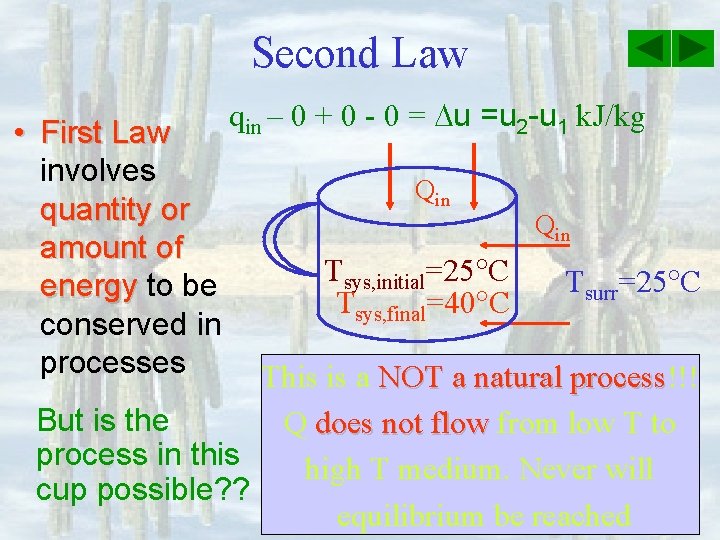
Second Law qin – 0 + 0 - 0 = u 2 - u 1, k. J/kg Qin Tsys, initial=25 C Tsys, final=40 C Qin Tsurr=25 C This is NOT a natural process!!! process Q does not flow from low T to high T medium. Never will the coffee return to its initial state.

Second Law qin – 0 + 0 - 0 = u 2 - u 1, k. J/kg Qin Tsys, initial=25 C Tsys, final=40 C Qin Tsurr=25 C This is NOT a natural process!!! process Q does not flow from low T to high T medium. Never will the coffee return to its initial state.

Second Law • First Law involves quantity or amount of energy to be conserved in processes qin – 0 + 0 - 0 = u =u 2 -u 1 k. J/kg Qin Tsys, initial=25 C Tsys, final=40 C Qin Tsurr=25 C This is a NOT a natural process!!! process But is the Q does not flow from low T to process in this high T medium. Never will cup possible? ? equilibrium be reached

Second Law • First Law is not sufficient to determine if a process can or cannot proceed

Second Law • First Law is not sufficient to determine if a process can or cannot proceed • Introduce the second law of thermodynamics – processes occur in its natural direction. Ø Heat (thermal energy) flows from high temperature medium to low temperature medium. Ø Energy has quality & quality is higher with higher temperature. More work can be done.

Second Law Considerations: • Work can be converted to heat directly & totally. • Heat cannot be converted to work directly & totally. Ø Requires a special device – heat engine.

Second Law Heat Engine Characteristics: • Receive heat from a high T source. • Convert part of the heat into work. • Reject excess heat into a low T sink. • Operates in a cycle.

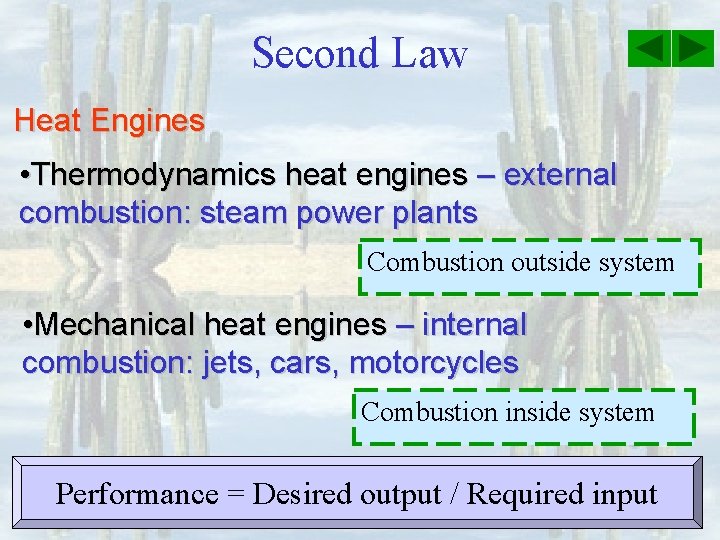
Second Law Heat Engines • Thermodynamics heat engines – external combustion: steam power plants Combustion outside system • Mechanical heat engines – internal combustion: jets, cars, motorcycles Combustion inside system Performance = Desired output / Required input

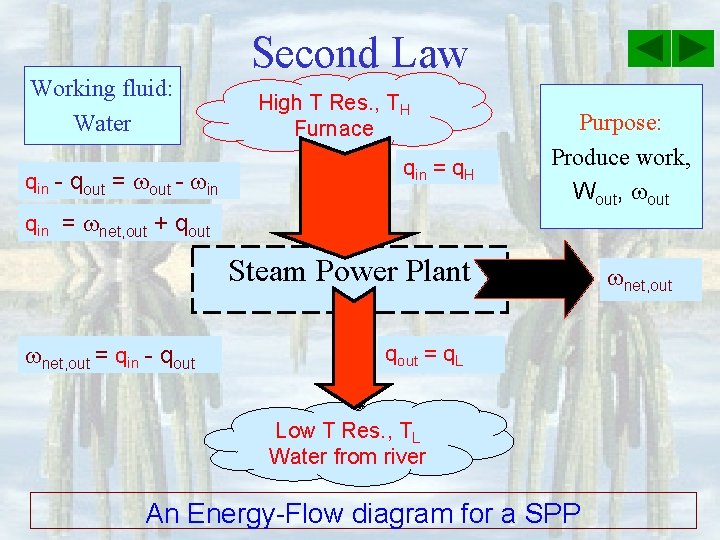
Working fluid: Water qin - qout = out - in Second Law High T Res. , TH Furnace qin = q. H qin = net, out + qout Purpose: Produce work, Wout, out Steam Power Plant net, out = qin - qout = q. L Low T Res. , TL Water from river An Energy-Flow diagram for a SPP net, out

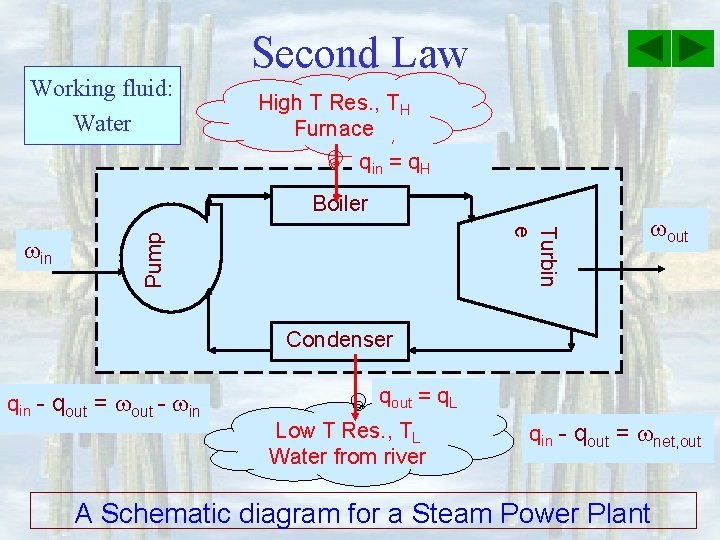
Working fluid: Water Second Law High T Res. , TH Furnace qin = q. H Boiler Pump Turbin e in out Condenser qin - qout = out - in qout = q. L Low T Res. , TL Water from river qin - qout = net, out A Schematic diagram for a Steam Power Plant

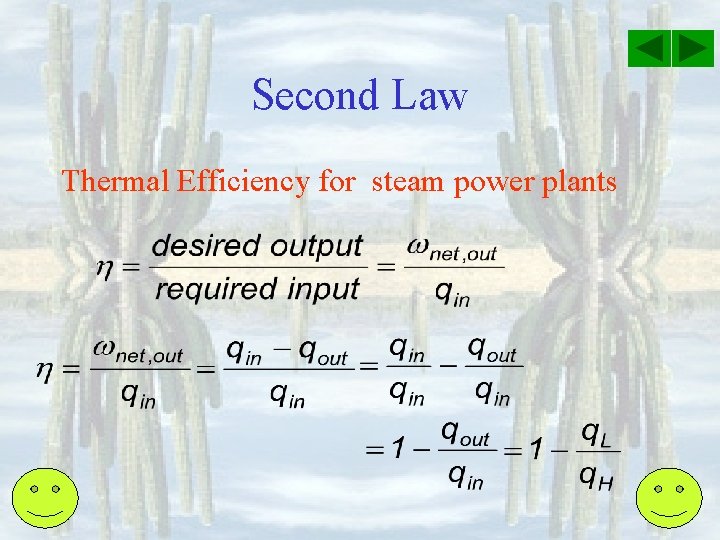
Second Law Thermal Efficiency for steam power plants

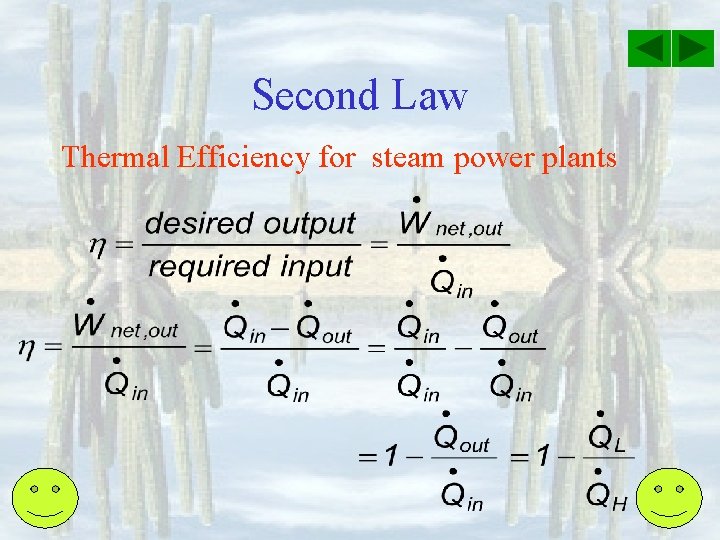
Second Law Thermal Efficiency for steam power plants

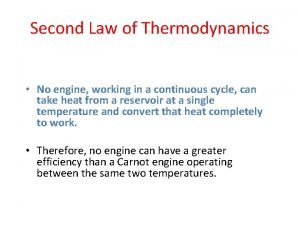
Second Law Kelvin Planck Statement for steam power plants • It is impossible for engines operating in a cycle to receive heat from a single reservoir and convert all of the heat into work. • Heat engines cannot be 100% efficient.

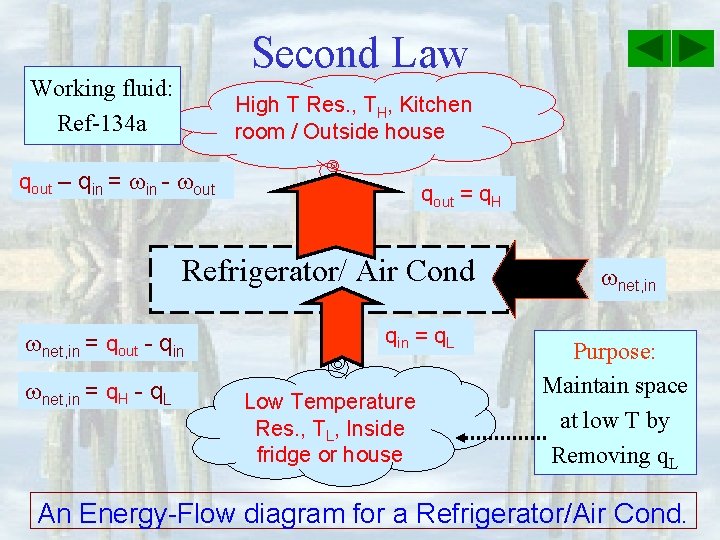
Second Law Working fluid: Ref-134 a High T Res. , TH, Kitchen room / Outside house qout – qin = in - out qout = q. H Refrigerator/ Air Cond net, in = qout - qin net, in = q. H - q. L qin = q. L Low Temperature Res. , TL, Inside fridge or house net, in Purpose: Maintain space at low T by Removing q. L An Energy-Flow diagram for a Refrigerator/Air Cond.

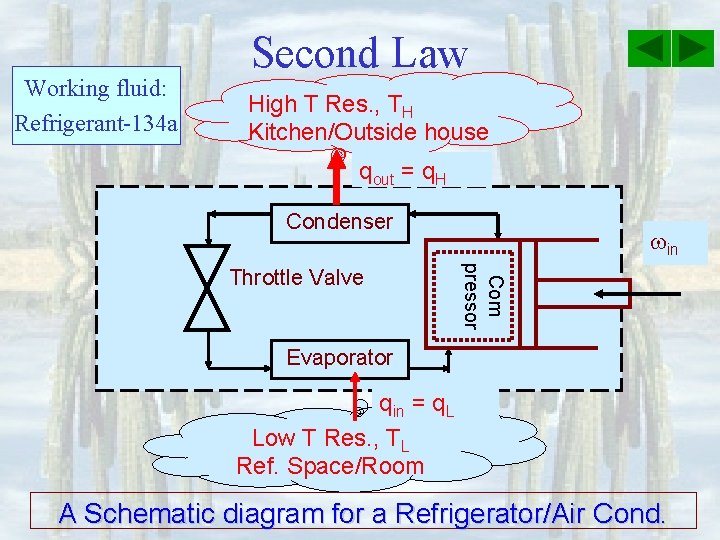
Working fluid: Refrigerant-134 a Second Law High T Res. , TH Kitchen/Outside house qout = q. H Condenser Com pressor Throttle Valve in Evaporator qin = q. L Low T Res. , TL Ref. Space/Room A Schematic diagram for a Refrigerator/Air Cond.

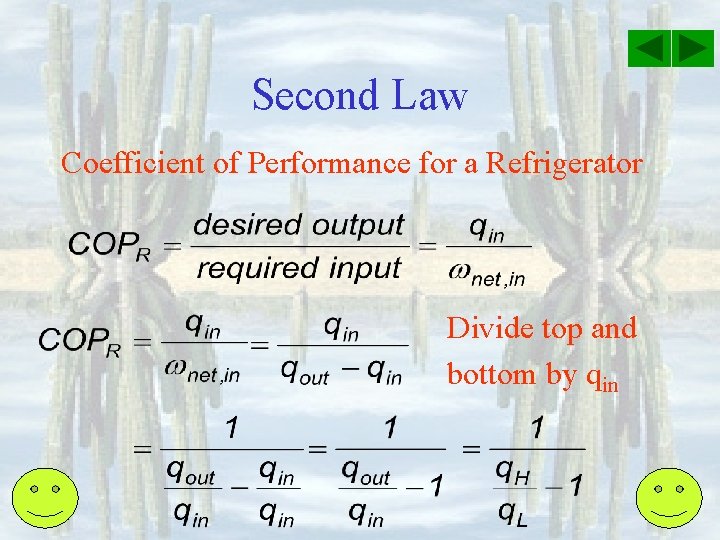
Second Law Coefficient of Performance for a Refrigerator Divide top and bottom by qin

Second Law Coefficient of Performance for a Refrigerator

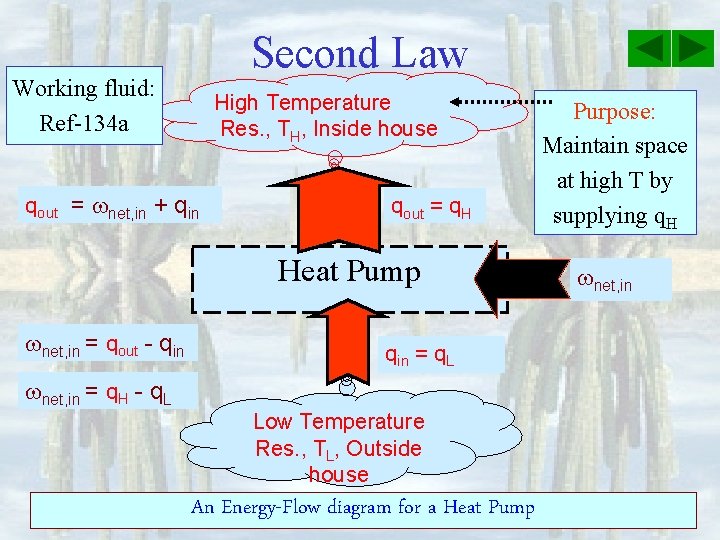
Second Law Working fluid: Ref-134 a High Temperature Res. , TH, Inside house qout = net, in + qin qout = q. H Heat Pump net, in = qout - qin = q. L net, in = q. H - q. L Low Temperature Res. , TL, Outside house An Energy-Flow diagram for a Heat Pump Purpose: Maintain space at high T by supplying q. H net, in

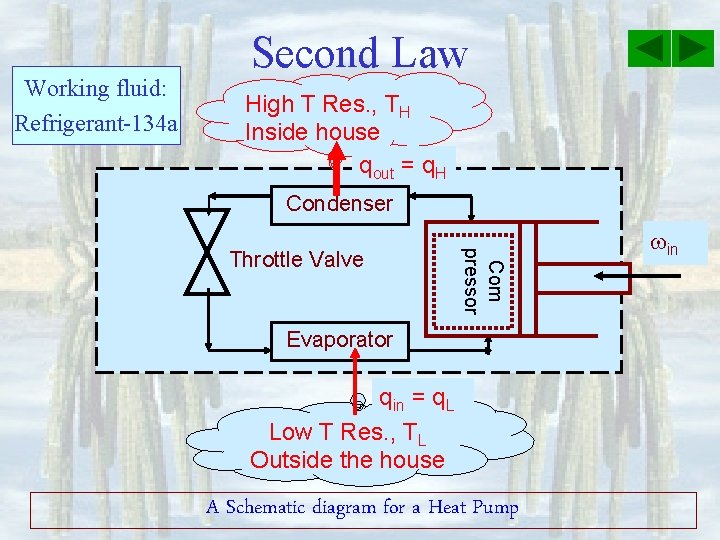
Working fluid: Refrigerant-134 a Second Law High T Res. , TH Inside house qout = q. H Condenser Com pressor Throttle Valve Evaporator qin = q. L Low T Res. , TL Outside the house A Schematic diagram for a Heat Pump in

Second Law Coefficient of Performance for a Heat Pump

Second Law Clausius Statement on Refrigerators/Heat Pump • It is impossible to construct a device operating in a cycle and produces no effect other than the transfer of heat from a low T to a high T medium. • Must do external work to the device to make it function. Hence more energy removed to the surrounding.

Second Law – Energy Degrade What is the maximum performance of real engines if it can never achieve 100%? ? Factors of irreversibilities • less heat can be converted to work – Friction between 2 moving surfaces – Processes happen too fast – Non-isothermal heat transfer

Second Law – Dream Engine Carnot Cycle • Isothermal expansion v. Slow adding of Q resulting in work done by system (system expand) v. Qin – Wout = U = 0. So, Qin = Wout. Pressure drops. • Adiabatic expansion v 0 – Wout = U. Final U smaller than initial U. v. T & P drops.

Second Law – Dream Engine Carnot Cycle • Isothermal compression v. Work done on the system v. Slow rejection of Q v- Qout + Win = U = 0. So, Qout = Win. v. Pressure increases. • Adiabatic compression v 0 + Win = U. Final U higher than initial U. v. T & P increases.

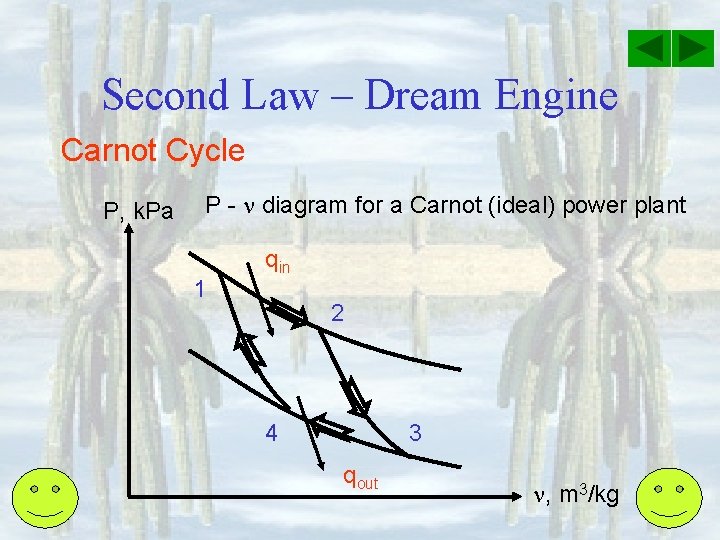
Second Law – Dream Engine Carnot Cycle P, k. Pa P - diagram for a Carnot (ideal) power plant 1 qin 2 4 3 qout , m 3/kg

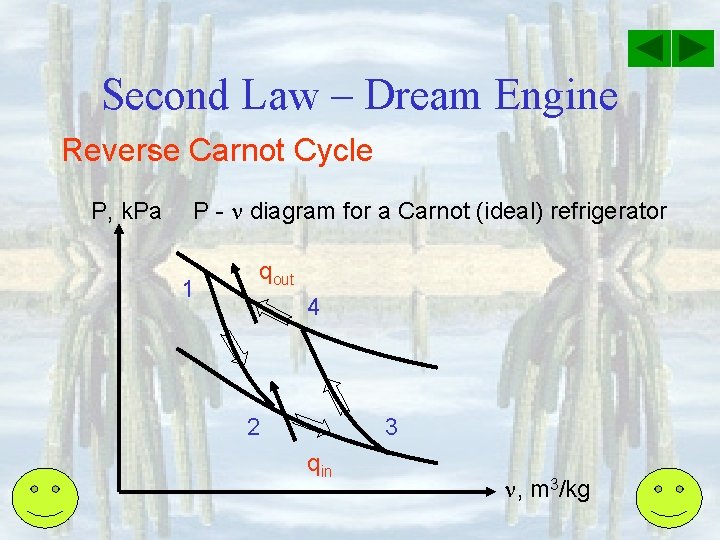
Second Law – Dream Engine Reverse Carnot Cycle P, k. Pa P - diagram for a Carnot (ideal) refrigerator 1 qout 4 2 3 qin , m 3/kg

Second Law – Dream Engine Carnot Principles • For heat engines in contact with the same hot and cold reservoir v. All reversible engines have the same performance. v. Real engines will have lower performance than the ideal engines.

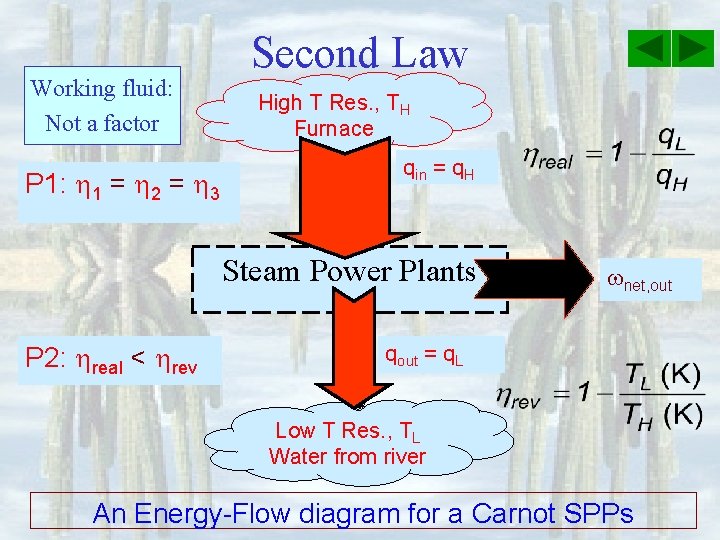
Working fluid: Not a factor P 1: 1 = 2 = 3 Second Law High T Res. , TH Furnace qin = q. H Steam Power Plants P 2: real < rev net, out qout = q. L Low T Res. , TL Water from river An Energy-Flow diagram for a Carnot SPPs

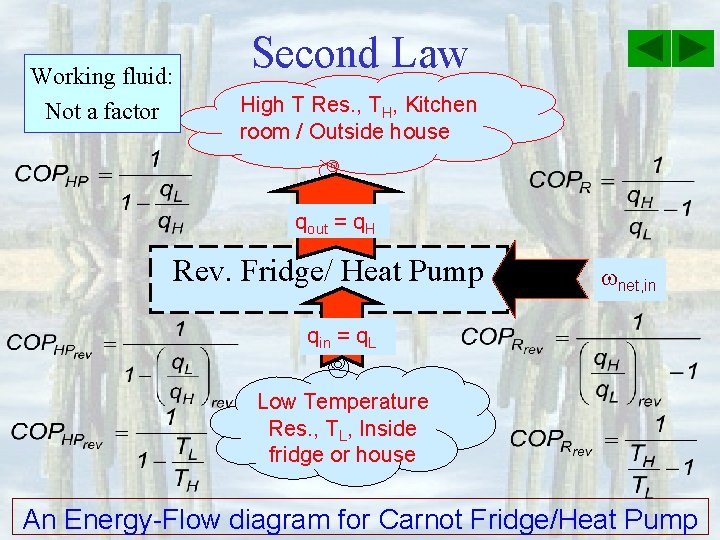
Working fluid: Not a factor Second Law High T Res. , TH, Kitchen room / Outside house qout = q. H Rev. Fridge/ Heat Pump net, in qin = q. L Low Temperature Res. , TL, Inside fridge or house An Energy-Flow diagram for Carnot Fridge/Heat Pump
 Specific entropy equation
Specific entropy equation State second law of thermodynamics
State second law of thermodynamics P v t relationship in adiabatic process
P v t relationship in adiabatic process Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics definition
Second law of thermodynamics definition Newton's first law and second law and third law
Newton's first law and second law and third law Si unit of newton's first law
Si unit of newton's first law 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad 186 282 miles per second into meters per second
186 282 miles per second into meters per second Geology lecture series
Geology lecture series Dcac lecture series
Dcac lecture series First law of thermodynamics open system
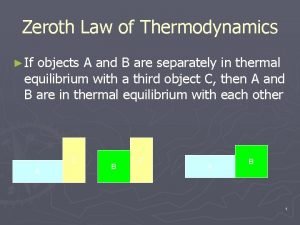
First law of thermodynamics open system Zeroth law of thermodynamics
Zeroth law of thermodynamics Newtons third law of thermodynamics
Newtons third law of thermodynamics Zeroth law of thermodynamics examples
Zeroth law of thermodynamics examples First law of thermodynamics
First law of thermodynamics First law of thermodynamics in cyclic process
First law of thermodynamics in cyclic process The joule experiment
The joule experiment 1st law of thermodynamics
1st law of thermodynamics Steady flow process thermodynamics
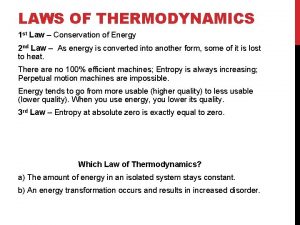
Steady flow process thermodynamics What are the laws of thermodynamics
What are the laws of thermodynamics Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Third law of thermodynamics is depend on
Third law of thermodynamics is depend on 1st law of thermodynamics
1st law of thermodynamics First law of thermodynamics sign convention
First law of thermodynamics sign convention 1st law of thermodynamics
1st law of thermodynamics 1st law of thermodynamics
1st law of thermodynamics First law of thermodynamics control mass
First law of thermodynamics control mass Zeroth law of thermodynamics
Zeroth law of thermodynamics Free energy
Free energy Zeroth law of thermodynamics
Zeroth law of thermodynamics Laws of thermo dynamics
Laws of thermo dynamics Frist law of thermodynamics
Frist law of thermodynamics Thermodynamics of ideal gases
Thermodynamics of ideal gases Boyle's law charles law avogadro's law
Boyle's law charles law avogadro's law Charles law constant
Charles law constant Project quality management lecture notes
Project quality management lecture notes Maclaurin series vs taylor series
Maclaurin series vs taylor series Balmer series lyman series
Balmer series lyman series Taylor vs maclaurin
Taylor vs maclaurin Taylor vs maclaurin
Taylor vs maclaurin Ibm p series
Ibm p series Feedback amplifier
Feedback amplifier Series aiding and series opposing
Series aiding and series opposing Sum of infinite series formula
Sum of infinite series formula Quality control and quality assurance
Quality control and quality assurance Pmp quality management
Pmp quality management Pmbok quality assurance vs quality control
Pmbok quality assurance vs quality control Total quality management seminar
Total quality management seminar Quality improvement vs quality assurance
Quality improvement vs quality assurance Concepts of quality
Concepts of quality Gurus of tqm
Gurus of tqm Crosby quality is free
Crosby quality is free What is tqm
What is tqm Second order integrated rate law
Second order integrated rate law Mendel's second law of independent assortment
Mendel's second law of independent assortment