Thermodynamics Lecture Series Ideal Rankine Cycle The Practical





![Vapor Cycle – Reheat Rankine Cycle Energy Analysis where s 6 = [sf +xsfg]@P Vapor Cycle – Reheat Rankine Cycle Energy Analysis where s 6 = [sf +xsfg]@P](https://slidetodoc.com/presentation_image_h/cd5f8e993329bfa2df924db6ea05cb2a/image-37.jpg)
- Slides: 39

Thermodynamics Lecture Series Ideal Rankine Cycle –The Practical Cycle Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@hotmail. com http: //www 5. uitm. edu. my/faculties/fsg/drjj 1. html

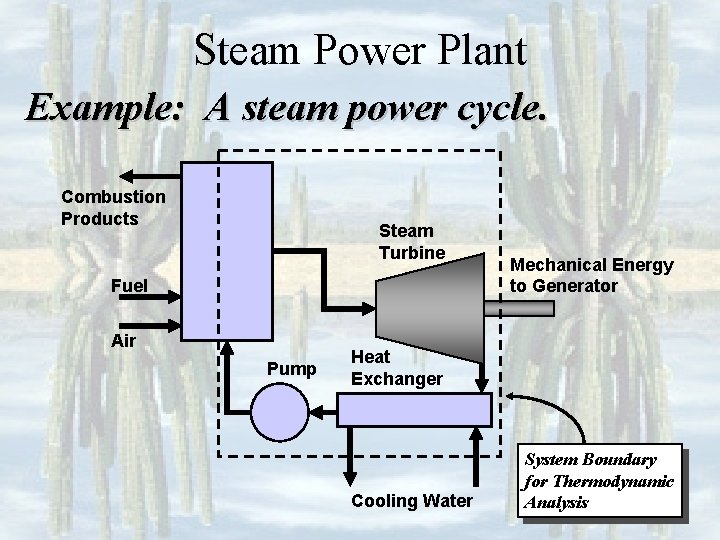
Steam Power Plant Example: A steam power cycle. Combustion Products Steam Turbine Fuel Air Pump Mechanical Energy to Generator Heat Exchanger Cooling Water System Boundary for Thermodynamic Analysis

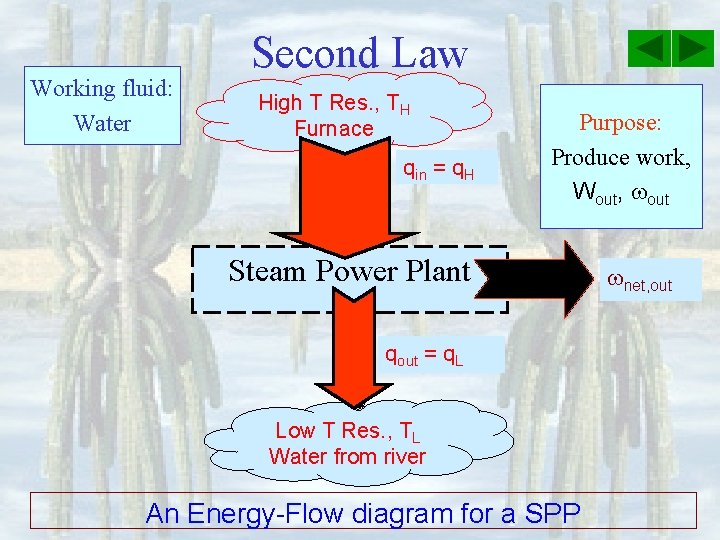
Working fluid: Water Second Law High T Res. , TH Furnace qin = q. H Purpose: Produce work, Wout, out Steam Power Plant qout = q. L Low T Res. , TL Water from river An Energy-Flow diagram for a SPP net, out

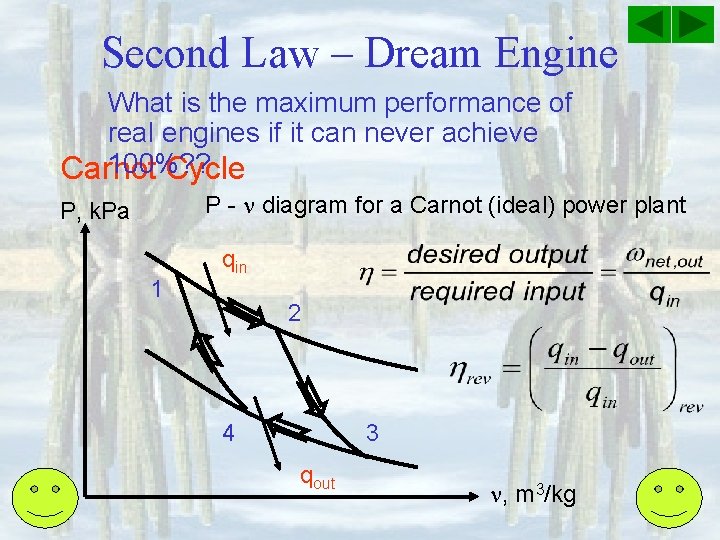
Second Law – Dream Engine What is the maximum performance of real engines if it can never achieve 100%? ? Carnot Cycle P - diagram for a Carnot (ideal) power plant P, k. Pa 1 qin 2 4 3 qout , m 3/kg

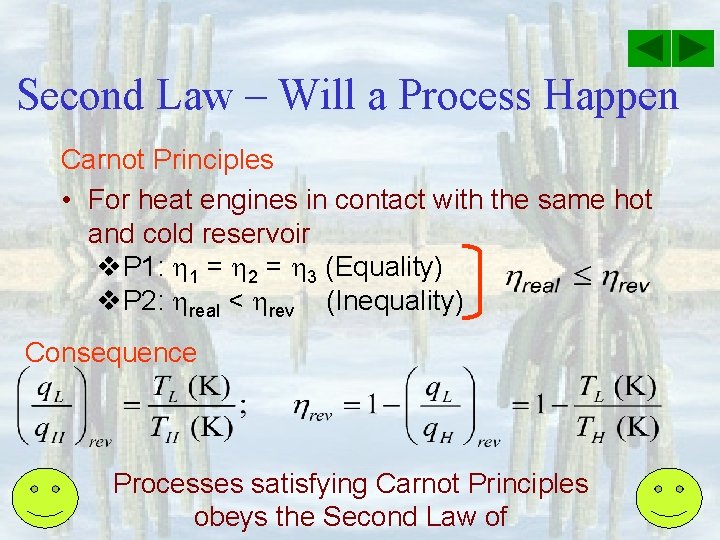
Second Law – Will a Process Happen Carnot Principles • For heat engines in contact with the same hot and cold reservoir v. P 1: 1 = 2 = 3 (Equality) v. P 2: real < rev (Inequality) Consequence Processes satisfying Carnot Principles obeys the Second Law of

Second Law – Will a Process Happen Clausius Inequality : • Sum of Q/T in a cyclic process must be zero for reversible processes and negative for real processes reversible impossible real

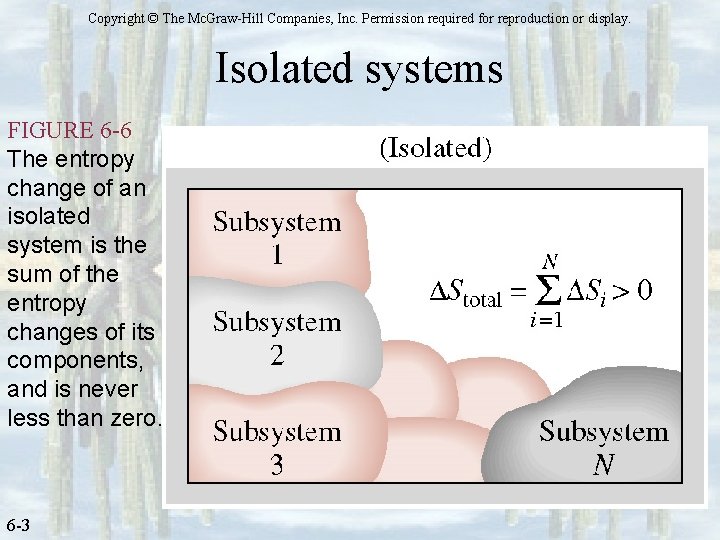
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Isolated systems FIGURE 6 -6 The entropy change of an isolated system is the sum of the entropy changes of its components, and is never less than zero. 6 -3

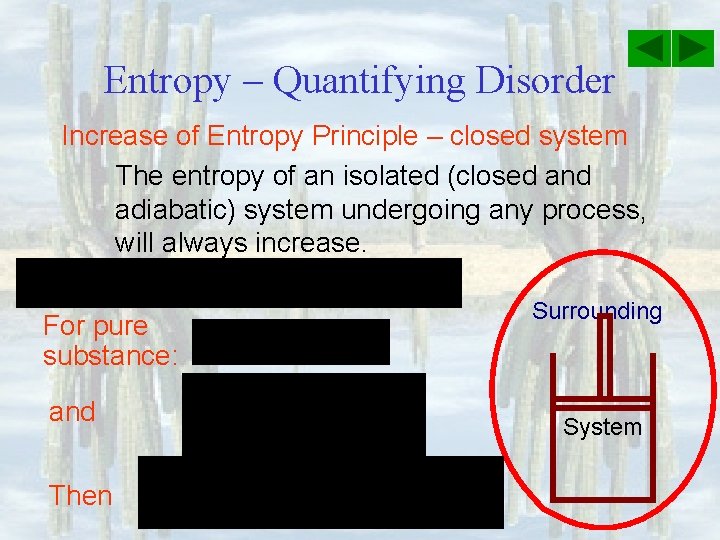
Entropy – Quantifying Disorder Increase of Entropy Principle – closed system The entropy of an isolated (closed and adiabatic) system undergoing any process, will always increase. For pure substance: and Then Surrounding System

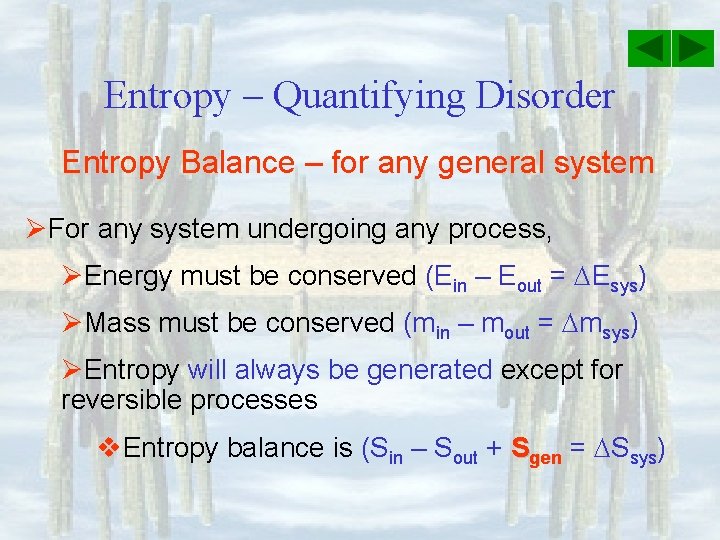
Entropy – Quantifying Disorder Entropy Balance – for any general system ØFor any system undergoing any process, ØEnergy must be conserved (Ein – Eout = Esys) ØMass must be conserved (min – mout = msys) ØEntropy will always be generated except for reversible processes v. Entropy balance is (Sin – Sout + Sgen = Ssys)

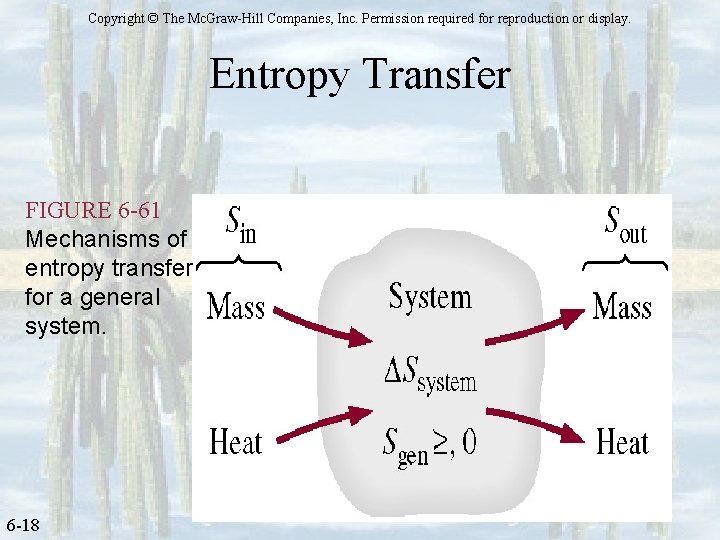
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Entropy Transfer FIGURE 6 -61 Mechanisms of entropy transfer for a general system. 6 -18

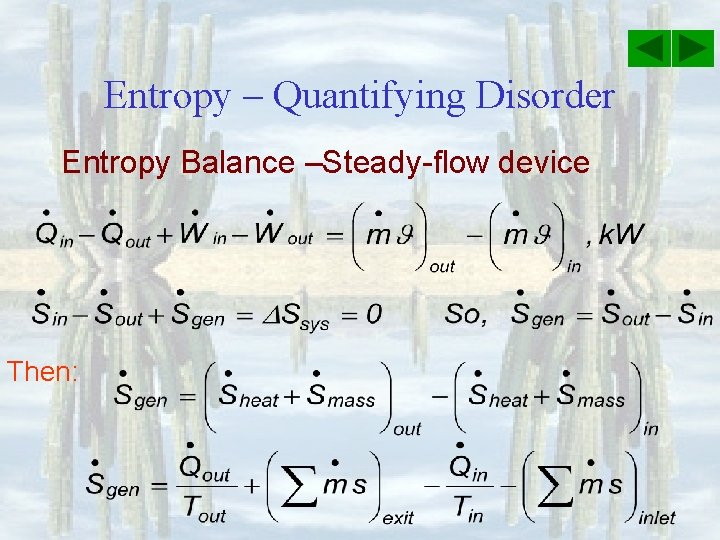
Entropy – Quantifying Disorder Entropy Balance –Steady-flow device Then:

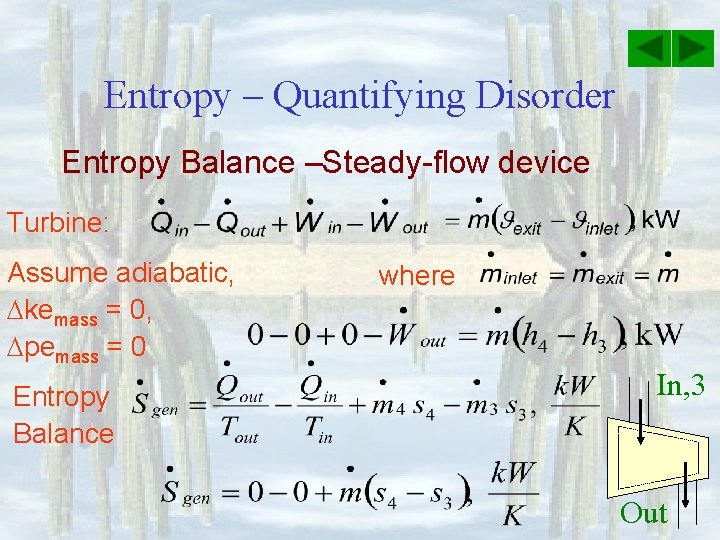
Entropy – Quantifying Disorder Entropy Balance –Steady-flow device Turbine: Assume adiabatic, kemass = 0, pemass = 0 Entropy Balance where In, 3 Out

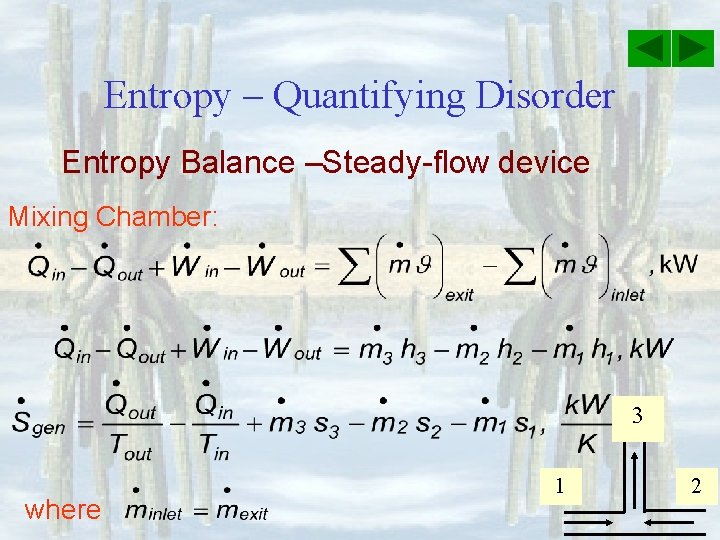
Entropy – Quantifying Disorder Entropy Balance –Steady-flow device Mixing Chamber: 3 where 1 2

Vapor Cycle Steam Power Plant ØExternal combustion ØFuel (q. H) from nuclear reactors, natural gas, charcoal ØWorking fluid is H 2 O Cheap, easily available & high enthalpy of vaporization hfg ØCycle is closed thermodynamic cycle ØAlternates between liquid and gas phase ØCan Carnot cycle be used for representing real SPP? ? ØAim: To decrease ratio of TL/TH

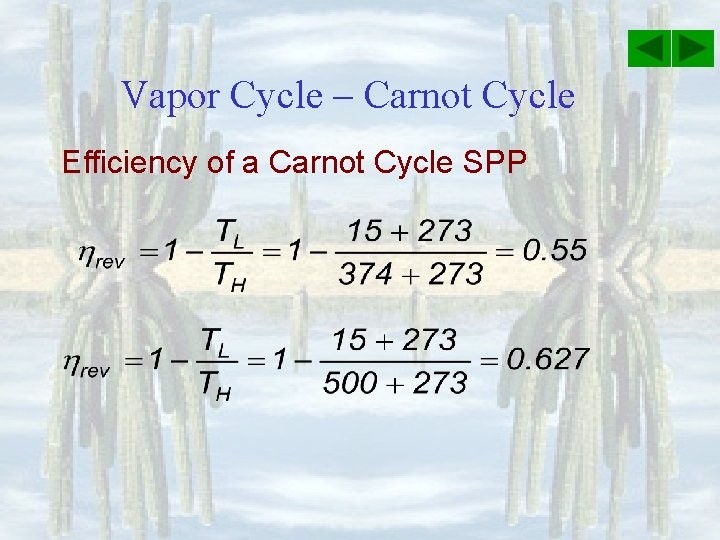
Vapor Cycle – Carnot Cycle Efficiency of a Carnot Cycle SPP

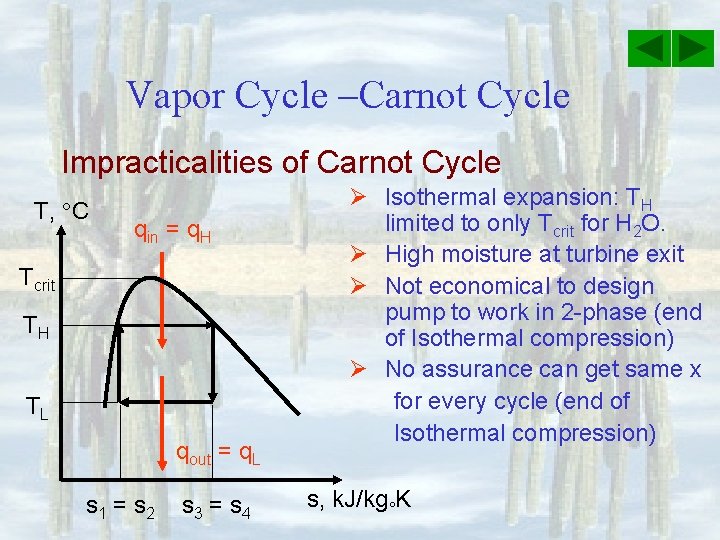
Vapor Cycle –Carnot Cycle Impracticalities of Carnot Cycle T, C qin = q. H Tcrit TH TL qout = q. L s 1 = s 2 s 3 = s 4 Ø Isothermal expansion: TH limited to only Tcrit for H 2 O. Ø High moisture at turbine exit Ø Not economical to design pump to work in 2 -phase (end of Isothermal compression) Ø No assurance can get same x for every cycle (end of Isothermal compression) s, k. J/kg K

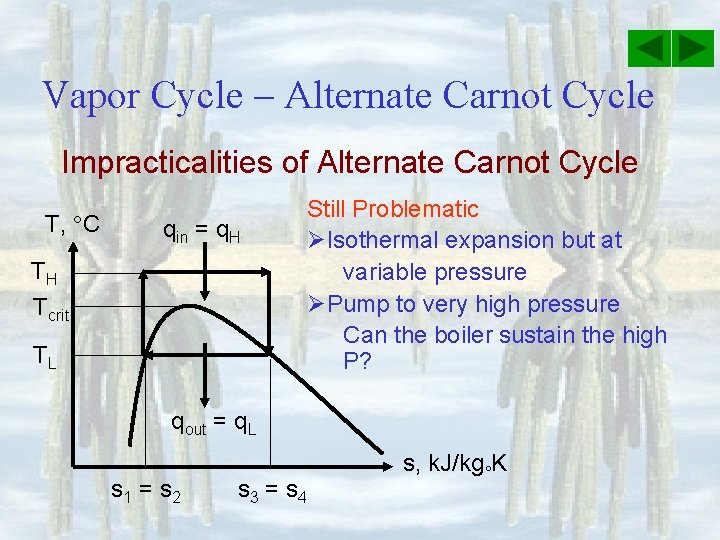
Vapor Cycle – Alternate Carnot Cycle Impracticalities of Alternate Carnot Cycle T, C qin = q. H TH Tcrit TL Still Problematic ØIsothermal expansion but at variable pressure ØPump to very high pressure Can the boiler sustain the high P? qout = q. L s 1 = s 2 s 3 = s 4 s, k. J/kg K

Vapor Cycle – Ideal Rankine Cycle Overcoming Impracticalities of Carnot Cycle ØSuperheat the H 2 O at a constant pressure (isobaric expansion) ü Can easily achieve desired TH higher than Tcrit. ü reduces moisture content at turbine exit ØRemove all excess heat at condenser ü Phase is sat. liquid at condenser exit, hence need only a pump to increase pressure ü Quality is zero for every cycle at condenser exit (pump inlet)

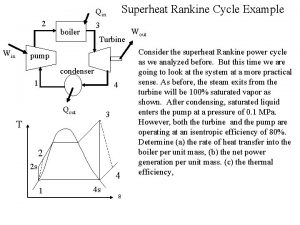
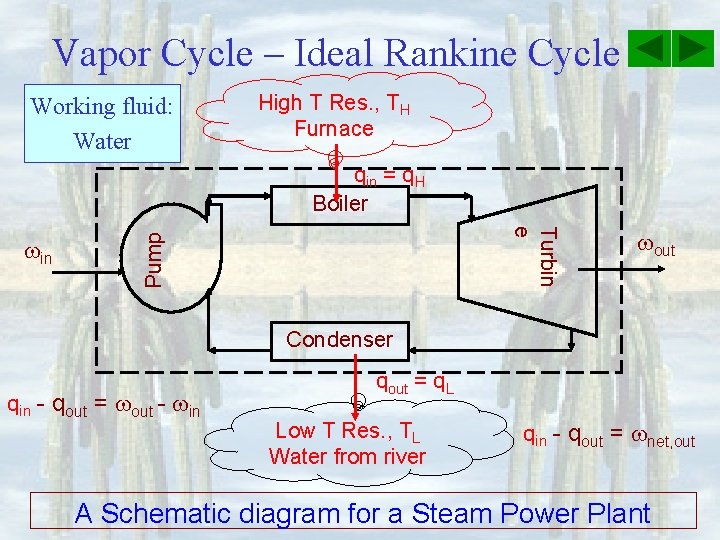
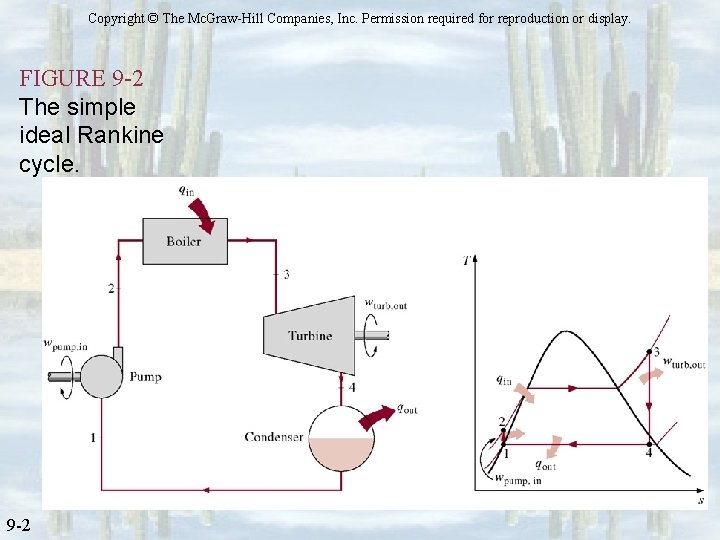
Vapor Cycle – Ideal Rankine Cycle Working fluid: Water High T Res. , TH Furnace qin = q. H Boiler Pump Turbin e in out Condenser qin - qout = out - in qout = q. L Low T Res. , TL Water from river qin - qout = net, out A Schematic diagram for a Steam Power Plant

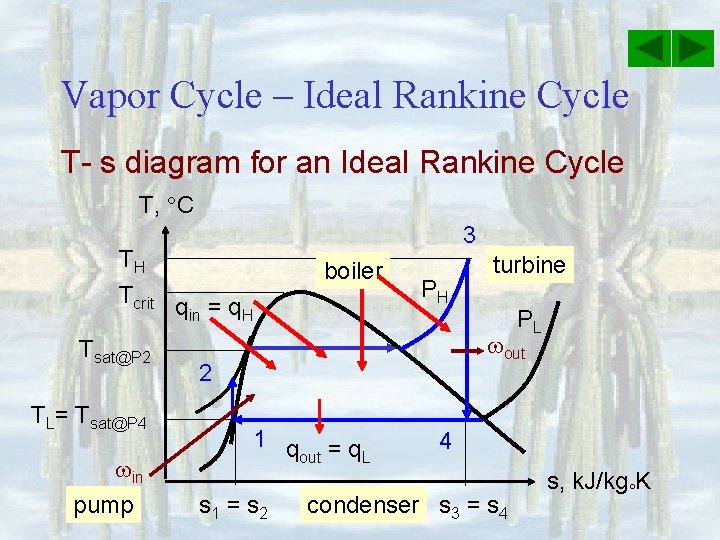
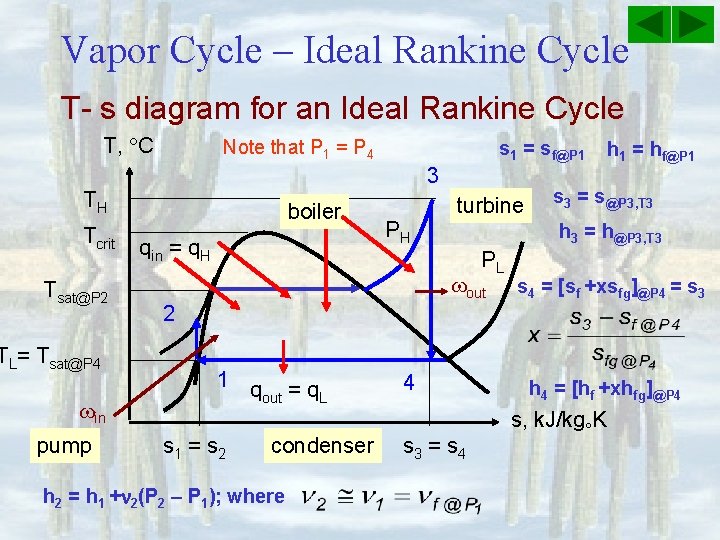
Vapor Cycle – Ideal Rankine Cycle T- s diagram for an Ideal Rankine Cycle T, C 3 TH Tcrit q = q in H Tsat@P 2 TL= Tsat@P 4 in pump boiler PH turbine PL out 2 1 q =q out L s 1 = s 2 4 condenser s 3 = s 4 s, k. J/kg K

Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. FIGURE 9 -2 The simple ideal Rankine cycle. 9 -2

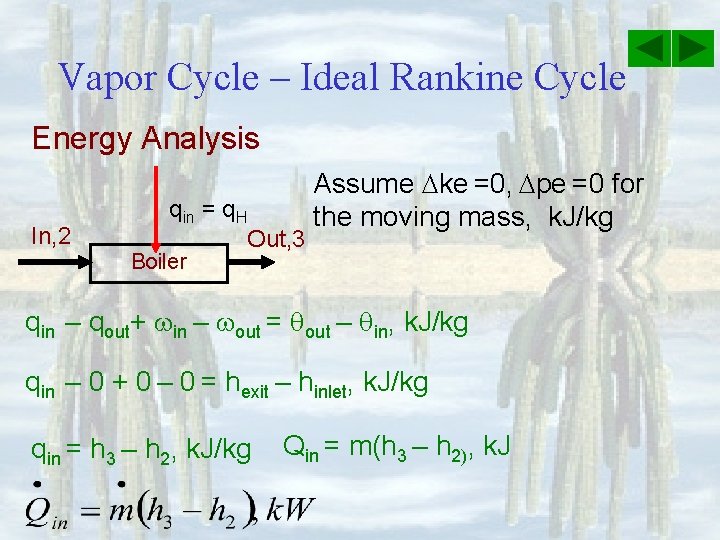
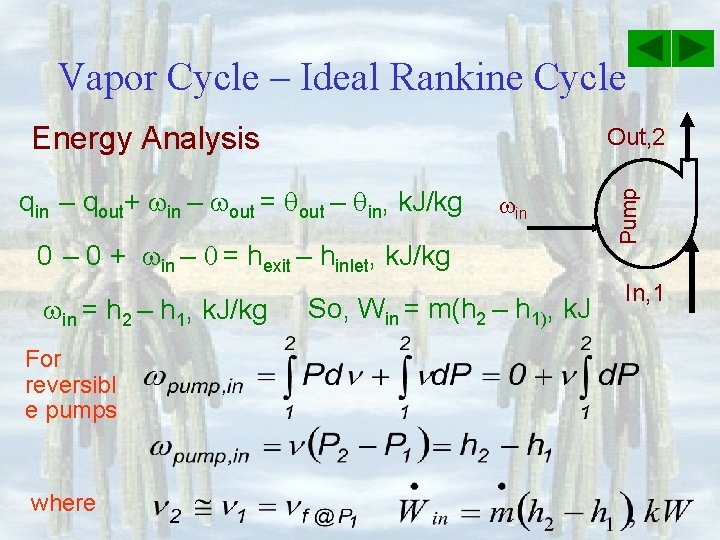
Vapor Cycle – Ideal Rankine Cycle Energy Analysis In, 2 qin = q. H Boiler Out, 3 Assume ke =0, pe =0 for the moving mass, k. J/kg qin – qout+ in – out = qout – qin, k. J/kg qin – 0 + 0 – 0 = hexit – hinlet, k. J/kg qin = h 3 – h 2, k. J/kg Qin = m(h 3 – h 2), k. J

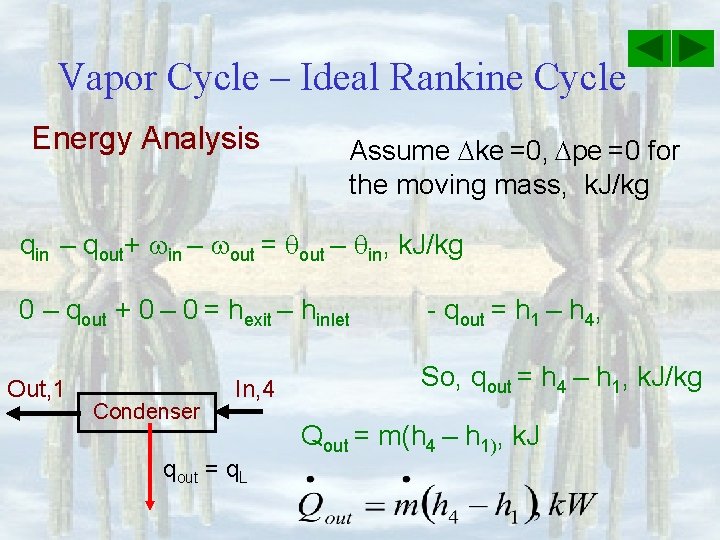
Vapor Cycle – Ideal Rankine Cycle Energy Analysis Assume ke =0, pe =0 for the moving mass, k. J/kg qin – qout+ in – out = qout – qin, k. J/kg 0 – qout + 0 – 0 = hexit – hinlet Out, 1 Condenser In, 4 qout = q. L - qout = h 1 – h 4, So, qout = h 4 – h 1, k. J/kg Qout = m(h 4 – h 1), k. J

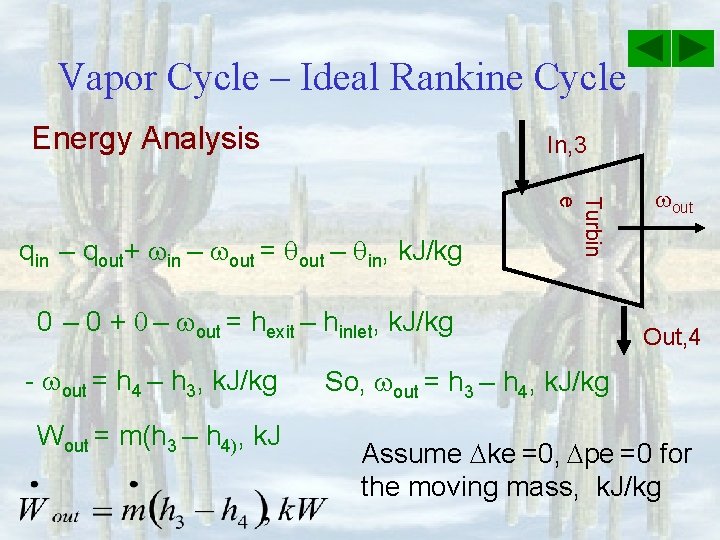
Vapor Cycle – Ideal Rankine Cycle Energy Analysis In, 3 Turbin e qin – qout+ in – out = qout – qin, k. J/kg 0 – 0 + 0 – out = hexit – hinlet, k. J/kg - out = h 4 – h 3, k. J/kg Wout = m(h 3 – h 4), k. J out Out, 4 So, out = h 3 – h 4, k. J/kg Assume ke =0, pe =0 for the moving mass, k. J/kg

Vapor Cycle – Ideal Rankine Cycle Energy Analysis qin – qout+ in – out = qout – qin, k. J/kg in 0 – 0 + in – 0 = hexit – hinlet, k. J/kg in = h 2 – h 1, k. J/kg For reversibl e pumps where So, Win = m(h 2 – h 1), k. J Pump Out, 2 In, 1

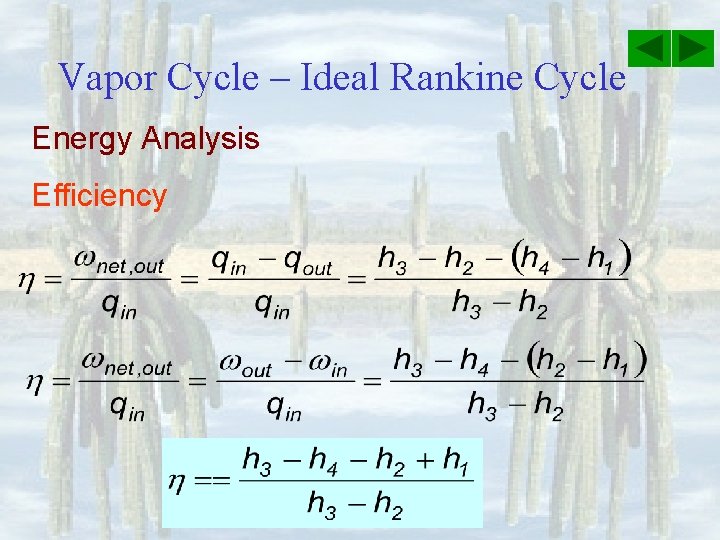
Vapor Cycle – Ideal Rankine Cycle Energy Analysis Efficiency

Vapor Cycle – Ideal Rankine Cycle T- s diagram for an Ideal Rankine Cycle T, C Note that P 1 = P 4 3 TH Tcrit Tsat@P 2 TL= Tsat@P 4 in pump s 1 = sf@P 1 boiler qin = q. H PH turbine h 1 = hf@P 1 s 3 = s@P 3, T 3 h 3 = h@P 3, T 3 PL out s 4 = [sf +xsfg]@P 4 = s 3 2 1 q =q out L s 1 = s 2 condenser h 2 = h 1 + 2(P 2 – P 1); where 4 s 3 = s 4 h 4 = [hf +xhfg]@P 4 s, k. J/kg K

Vapor Cycle – Ideal Rankine Cycle Energy Analysis Increasing Efficiency ØMust increase net, out = qin – qout üIncrease area under process cycle ØDecrease condenser pressure; P 1=P 4 üPmin > Psat@Tcooling+10 deg C ØSuperheat üT 3 limited to metullargical strength of boiler ØIncrease boiler pressure; P 2=P 3 üWill decrease quality (an increase in moisture). Minimum x is 89. 6%.

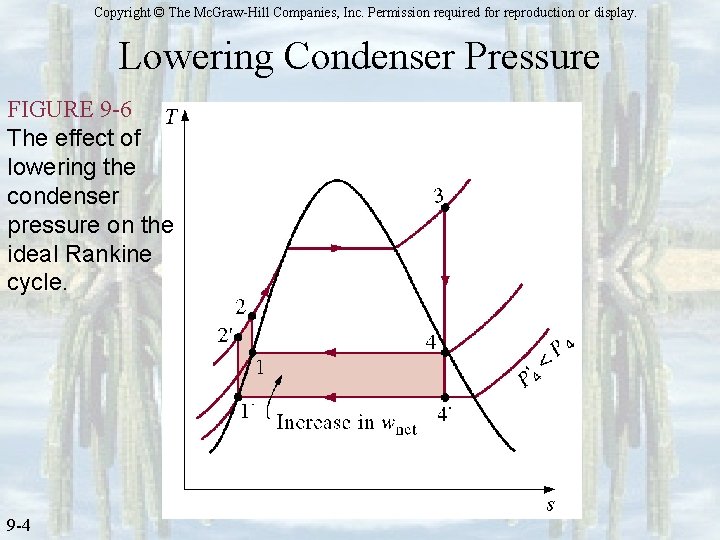
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Lowering Condenser Pressure FIGURE 9 -6 The effect of lowering the condenser pressure on the ideal Rankine cycle. 9 -4

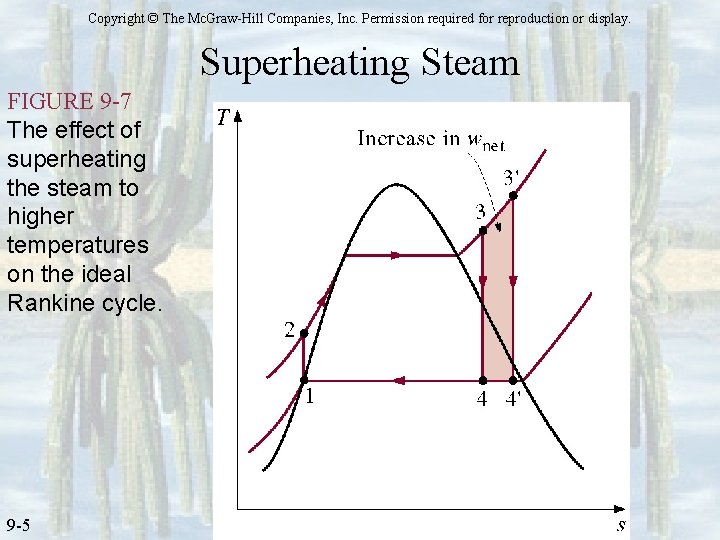
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Superheating Steam FIGURE 9 -7 The effect of superheating the steam to higher temperatures on the ideal Rankine cycle. 9 -5

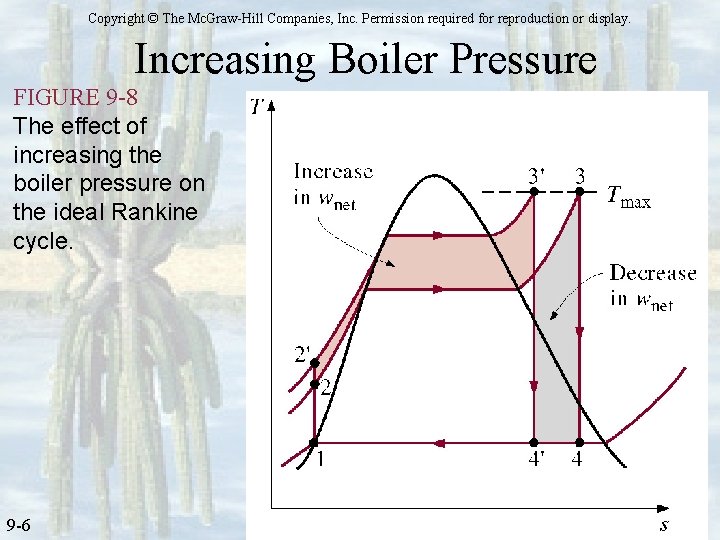
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Increasing Boiler Pressure FIGURE 9 -8 The effect of increasing the boiler pressure on the ideal Rankine cycle. 9 -6

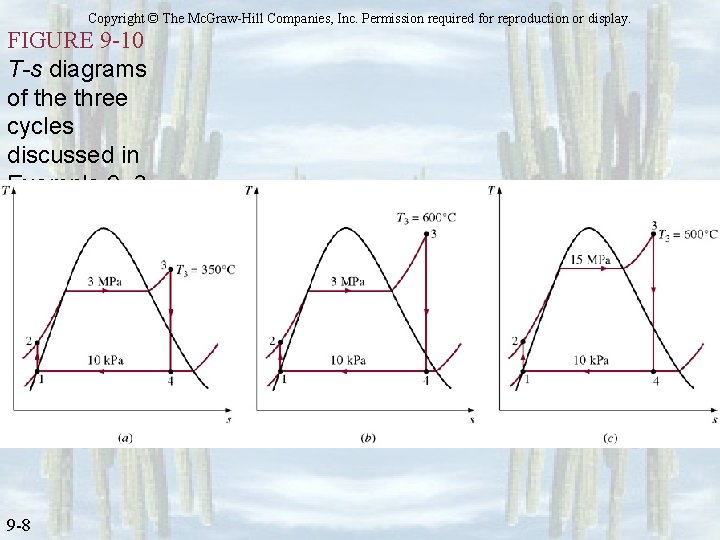
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. FIGURE 9 -10 T-s diagrams of the three cycles discussed in Example 9– 3. 9 -8

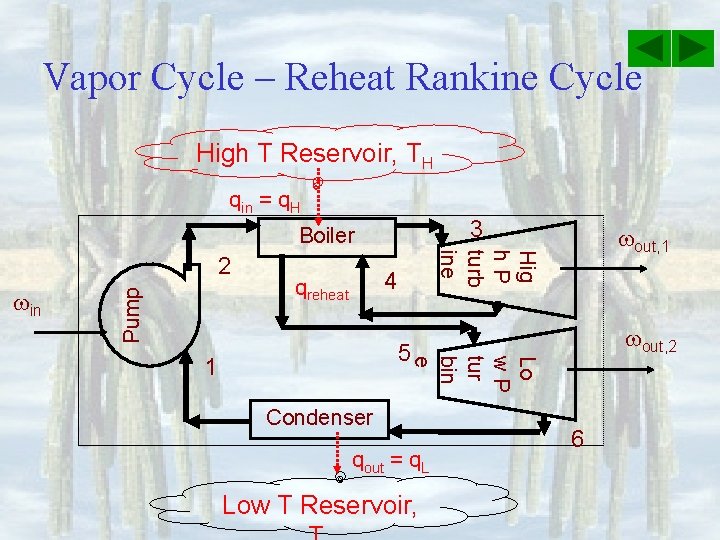
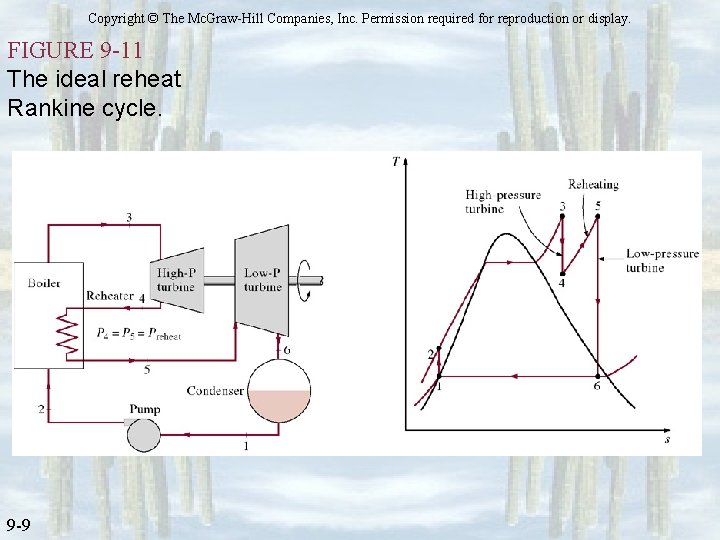
Vapor Cycle – Reheat Rankine Cycle High T Reservoir, TH qin = q. H 3 Boiler Pump in Hig h. P turb ine 2 4 qreheat out, 1 out, 2 5 Lo w. P tur bin e 1 Condenser qout = q. L Low T Reservoir, 6

Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. FIGURE 9 -11 The ideal reheat Rankine cycle. 9 -9

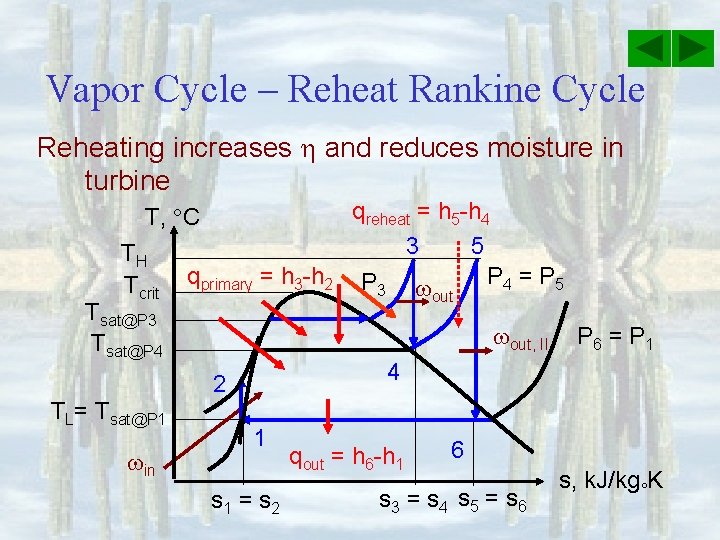
Vapor Cycle – Reheat Rankine Cycle Reheating increases and reduces moisture in turbine T, C TH Tcrit qprimary = h 3 -h 2 Tsat@P 3 Tsat@P 4 in out, II P 6 = P 1 4 2 TL= Tsat@P 1 qreheat = h 5 -h 4 5 3 P 4 = P 5 P 3 1 s 1 = s 2 qout = h 6 -h 1 6 s 3 = s 4 s 5 = s 6 s, k. J/kg K

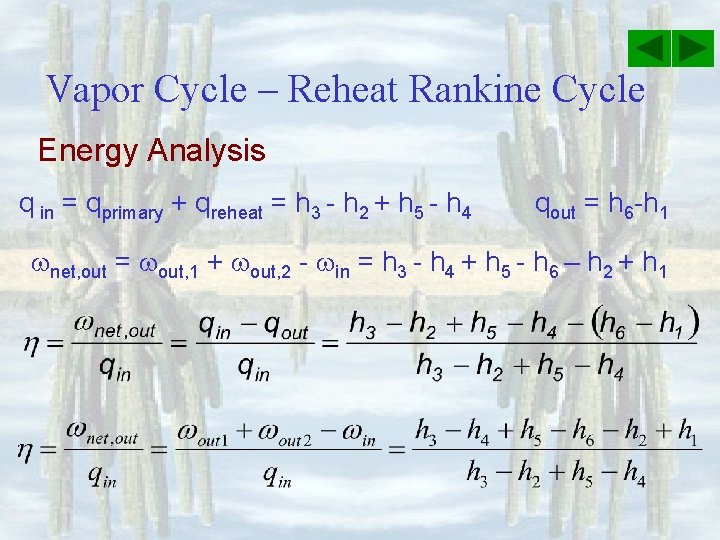
Vapor Cycle – Reheat Rankine Cycle Energy Analysis q in = qprimary + qreheat = h 3 - h 2 + h 5 - h 4 qout = h 6 -h 1 net, out = out, 1 + out, 2 - in = h 3 - h 4 + h 5 - h 6 – h 2 + h 1
![Vapor Cycle Reheat Rankine Cycle Energy Analysis where s 6 sf xsfgP Vapor Cycle – Reheat Rankine Cycle Energy Analysis where s 6 = [sf +xsfg]@P](https://slidetodoc.com/presentation_image_h/cd5f8e993329bfa2df924db6ea05cb2a/image-37.jpg)
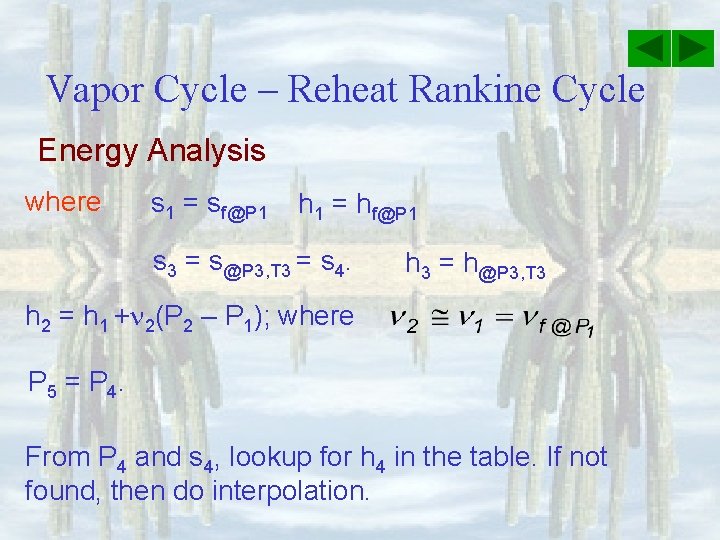
Vapor Cycle – Reheat Rankine Cycle Energy Analysis where s 6 = [sf +xsfg]@P 6. Use x = 0. 896 and s 5 = s 6 h 6 = [hf +xhfg]@P 6 Knowing s 5 and T 5, P 5 needs to be estimated (usually approximately a quarter of P 3 to ensure x is around 89%. On the property table, choose P 5 so that the entropy is lower than s 5 above. Then can find h 5 = h@P 5, T 5.

Vapor Cycle – Reheat Rankine Cycle Energy Analysis where s 1 = sf@P 1 h 1 = hf@P 1 s 3 = s@P 3, T 3 = s 4. h 3 = h@P 3, T 3 h 2 = h 1 + 2(P 2 – P 1); where P 5 = P 4. From P 4 and s 4, lookup for h 4 in the table. If not found, then do interpolation.

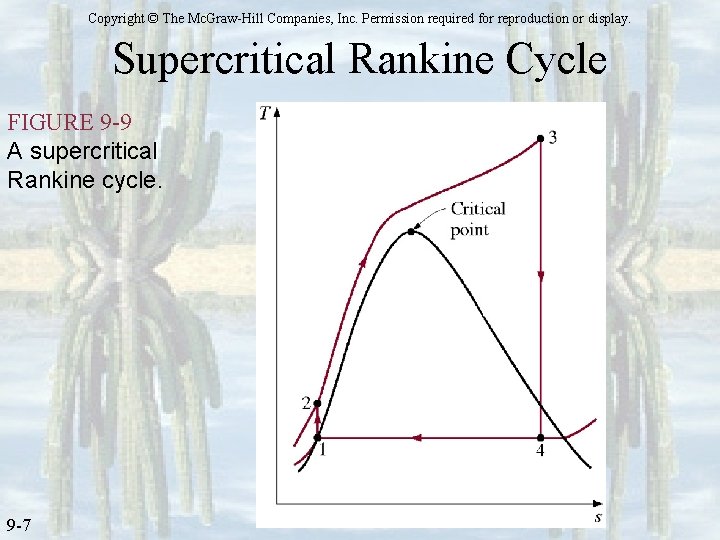
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Supercritical Rankine Cycle FIGURE 9 -9 A supercritical Rankine cycle. 9 -7
 Qin thermodynamics
Qin thermodynamics Rankine ideal
Rankine ideal Ideal ara ısıtmalı rankine çevrimi
Ideal ara ısıtmalı rankine çevrimi Ara buhar almalı rankine çevrimi
Ara buhar almalı rankine çevrimi Rankine cycle efficiency
Rankine cycle efficiency Superheated rankine cycle
Superheated rankine cycle Cordinal points
Cordinal points Rankine cycle efficiency
Rankine cycle efficiency Steam cycle
Steam cycle Verdicorp
Verdicorp Heat addition in rankine cycle
Heat addition in rankine cycle Verdicorp
Verdicorp Rankine cycle reheat
Rankine cycle reheat Brayton cycle with regeneration problems
Brayton cycle with regeneration problems Reinforcement lap lengths eurocodes
Reinforcement lap lengths eurocodes Ideal solution thermodynamics
Ideal solution thermodynamics First law of thermodynamics
First law of thermodynamics 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Difference between ideal and practical transformer
Difference between ideal and practical transformer Comparison between ideal and practical op amp
Comparison between ideal and practical op amp Transformner
Transformner Feedback ideal
Feedback ideal Multipurpose refrigeration systems with a single compressor
Multipurpose refrigeration systems with a single compressor What is brayton cycle in thermodynamics
What is brayton cycle in thermodynamics What is brayton cycle in thermodynamics
What is brayton cycle in thermodynamics What is brayton cycle in thermodynamics
What is brayton cycle in thermodynamics Cycle efficiency is defined as....
Cycle efficiency is defined as.... Geology lecture series
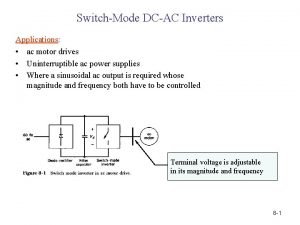
Geology lecture series Dcac lecture series
Dcac lecture series Slenderness ratio formula
Slenderness ratio formula Passive earth pressure formula
Passive earth pressure formula Théorie de rankine
Théorie de rankine Rankine
Rankine Cycle de rankin
Cycle de rankin Rankine
Rankine Teoria di rankine
Teoria di rankine Rankine and coulomb's theory of lateral earth pressure
Rankine and coulomb's theory of lateral earth pressure Rankine scale
Rankine scale Immiscible
Immiscible Maclaurin series vs taylor series
Maclaurin series vs taylor series