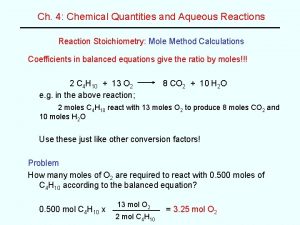
The use of EPR in Nitric Oxide Research












- Slides: 44

The use of EPR in Nitric Oxide Research Neil Hogg, Medical College of Wisconsin SFRBM 2005 Austin, TX

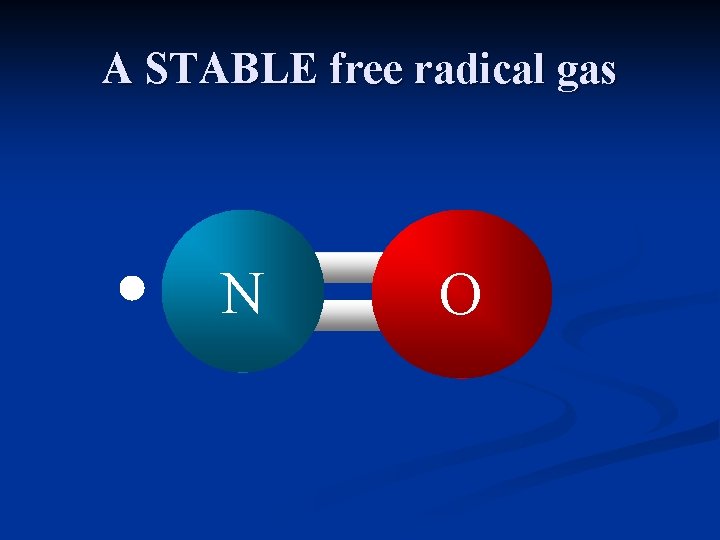
A STABLE free radical gas N O

Direct Detection of NO by EPR Broad ugly looking spectrum n Need high concentration n Unsuitable for biological detection n → Spin ‘Trapping’

Strategies used for the EPR detection of Nitric Oxide Fe/ Dithiocarbamate Nitronyl Nitroxides Hemoglobin/ Myoglobin DNIC

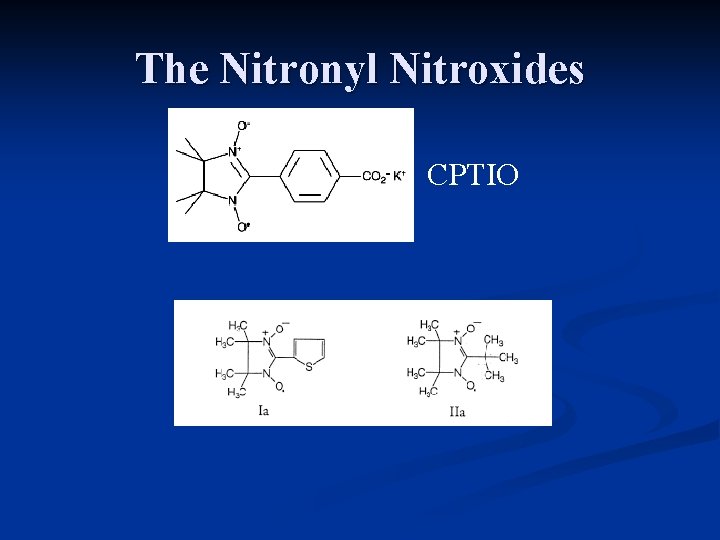
The Nitronyl Nitroxides CPTIO

Brief History First reported by Osieki and Ullman (1968) JACS, 90, 1078 n Proposed use as ‘NO dosimeter’ by Nadeau and Boocock (1977) Anal. Chem. 49, 1672 n Role as Biological NO spin trap. Joseph et al (1993), BBRC, 192, 926 n Antagonism of EDRF. Akaike et al (1993) Biochemistry. 32, 827 n

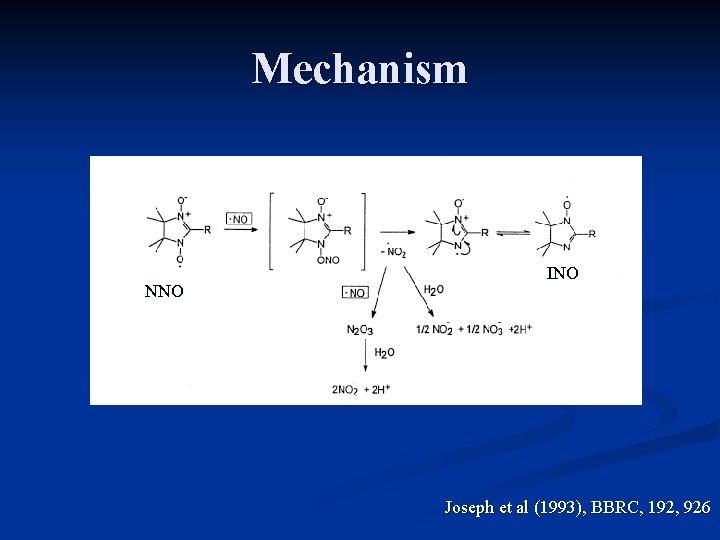
Mechanism NNO INO Joseph et al (1993), BBRC, 192, 926

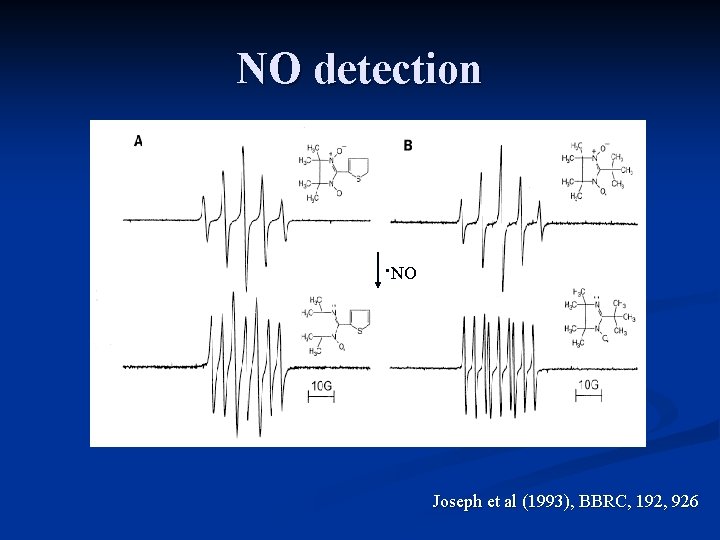
NO detection ∙NO Joseph et al (1993), BBRC, 192, 926

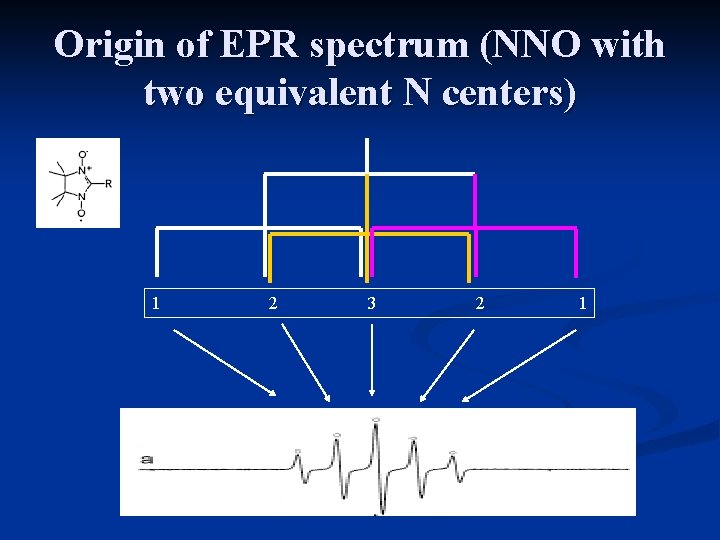
Origin of EPR spectrum (NNO with two equivalent N centers) 1 2 3 2 1

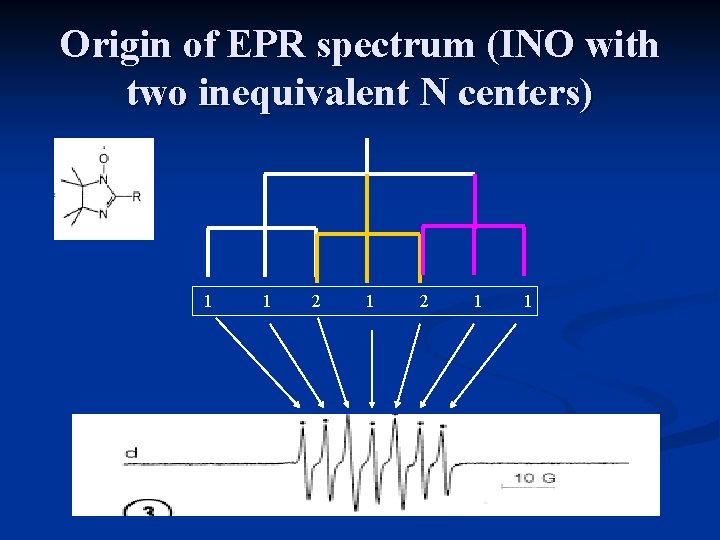
Origin of EPR spectrum (INO with two inequivalent N centers) 1 1 2 1 1

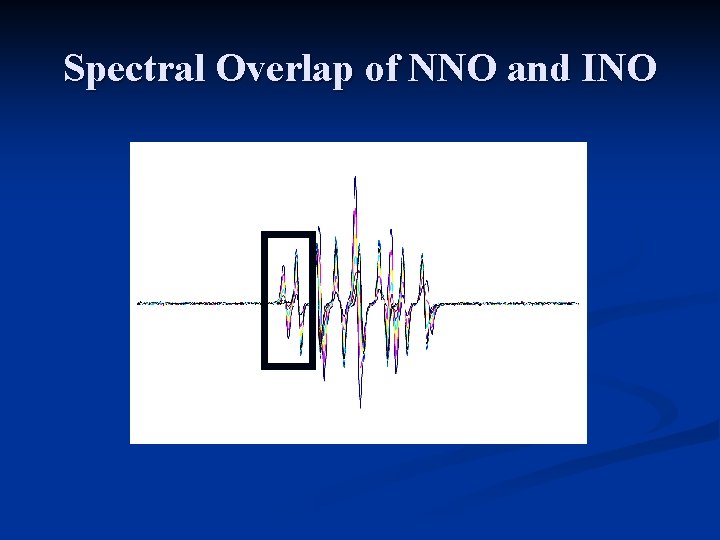
Spectral Overlap of NNO and INO

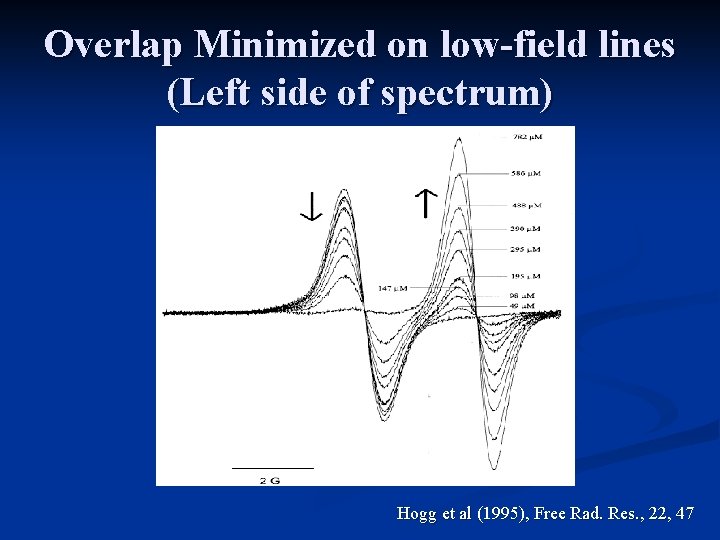
Overlap Minimized on low-field lines (Left side of spectrum) Hogg et al (1995), Free Rad. Res. , 22, 47

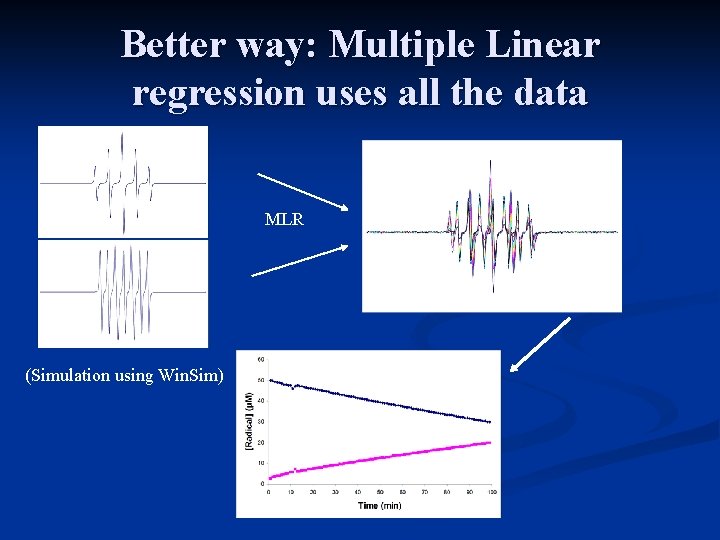
Better way: Multiple Linear regression uses all the data MLR (Simulation using Win. Sim)

Reaction characteristics: n n n Reaction of NO converts one radical to anotherefore not spin-trapping in the traditional sense. Rate const of ~1000 M-1 s-1 therefore fast enough to compete with oxygen but not fast enough to compete with (e. g. ) superoxide. Cannot use the ‘DMPO’ trick of using huge amounts of trap to offset a small rate constant due to the fact that the trap itself has an EPR spectrum

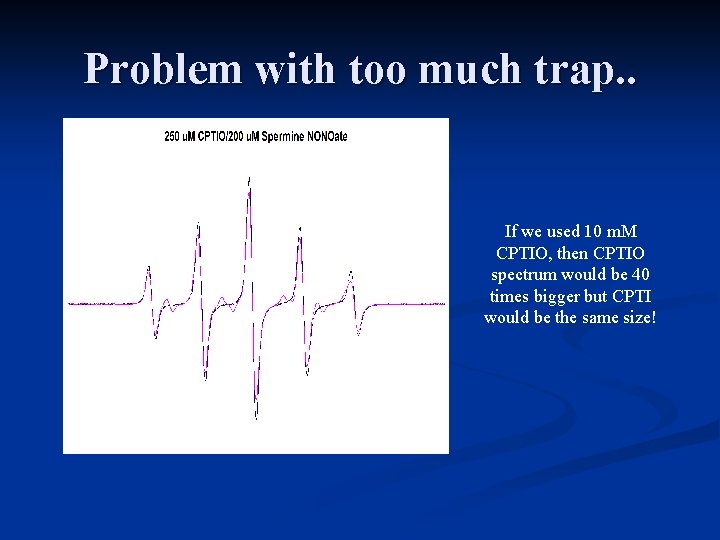
Problem with too much trap. . If we used 10 m. M CPTIO, then CPTIO spectrum would be 40 times bigger but CPTI would be the same size!

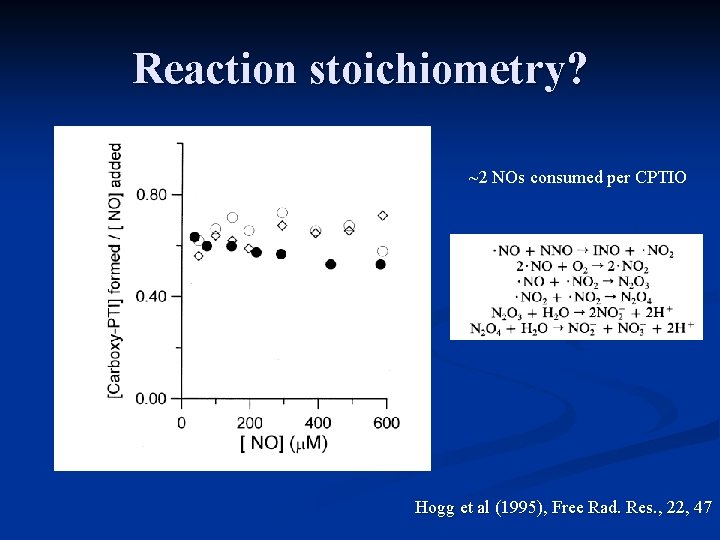
Reaction stoichiometry? ~2 NOs consumed per CPTIO Hogg et al (1995), Free Rad. Res. , 22, 47

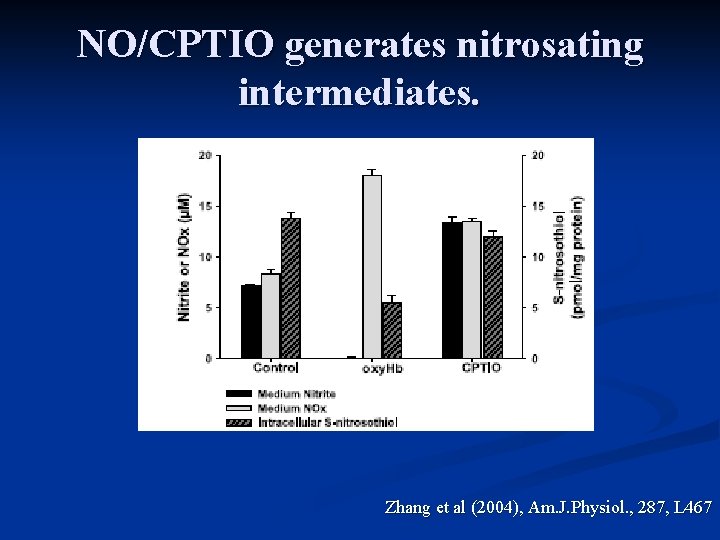
NO/CPTIO generates nitrosating intermediates. Zhang et al (2004), Am. J. Physiol. , 287, L 467

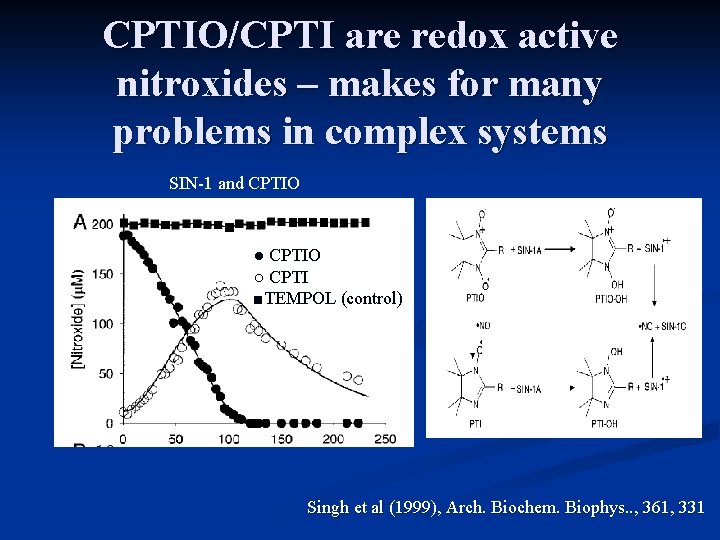
CPTIO/CPTI are redox active nitroxides – makes for many problems in complex systems SIN-1 and CPTIO ● CPTIO ○ CPTI ■TEMPOL (control) Singh et al (1999), Arch. Biochem. Biophys. . , 361, 331

Advantages/Disadvantages n n Clear NO-dependent change in EPR spectrum allows quantification of kinetics of NO formation. Works best in simple chemical systems as both reactant and product nitroxides are easily reduced by cellular reductants. The nitroxides are good oxidants and so care must be taken to examine if the redox properties of the nitroxides are altering the chemistry of the system Nitrogen dioxide is a product of the reaction and so these compounds my inhibit NO but enhance nitrosation/nitration reactions.

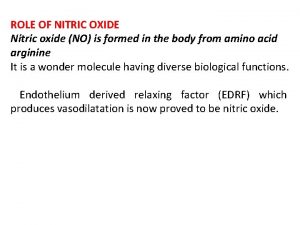
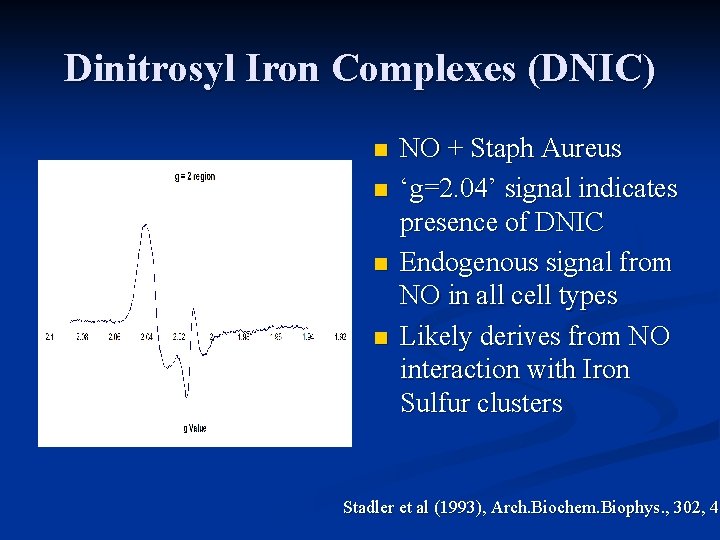
Dinitrosyl Iron Complexes (DNIC) n n NO + Staph Aureus ‘g=2. 04’ signal indicates presence of DNIC Endogenous signal from NO in all cell types Likely derives from NO interaction with Iron Sulfur clusters Stadler et al (1993), Arch. Biochem. Biophys. , 302, 4

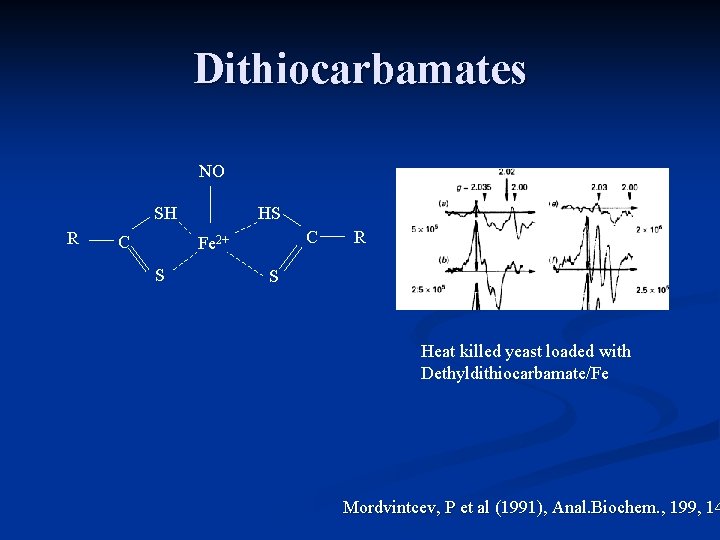
Dithiocarbamates NO SH R C HS C Fe 2+ S R S Heat killed yeast loaded with Dethyldithiocarbamate/Fe Mordvintcev, P et al (1991), Anal. Biochem. , 199, 14

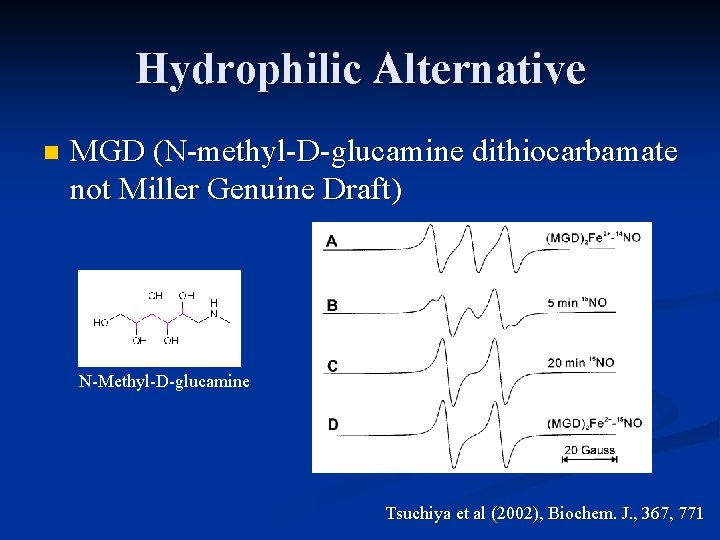
Hydrophilic Alternative n MGD (N-methyl-D-glucamine dithiocarbamate not Miller Genuine Draft) N-Methyl-D-glucamine Tsuchiya et al (2002), Biochem. J. , 367, 771

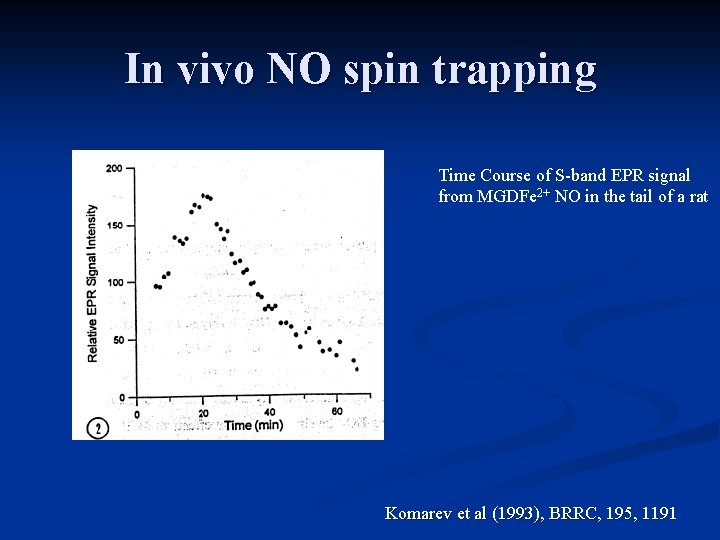
In vivo NO spin trapping Time Course of S-band EPR signal from MGDFe 2+ NO in the tail of a rat Komarev et al (1993), BRRC, 195, 1191

EPR imaging of NO using MGD Spatial mapping of nitric oxide generation in the ischemic heart using electron paramagnetic resonance imaging. Kuppusamy P, Wang P, Samouilov A, Zweier JL. Magn Reson Med. 1996 36: 212 -8.

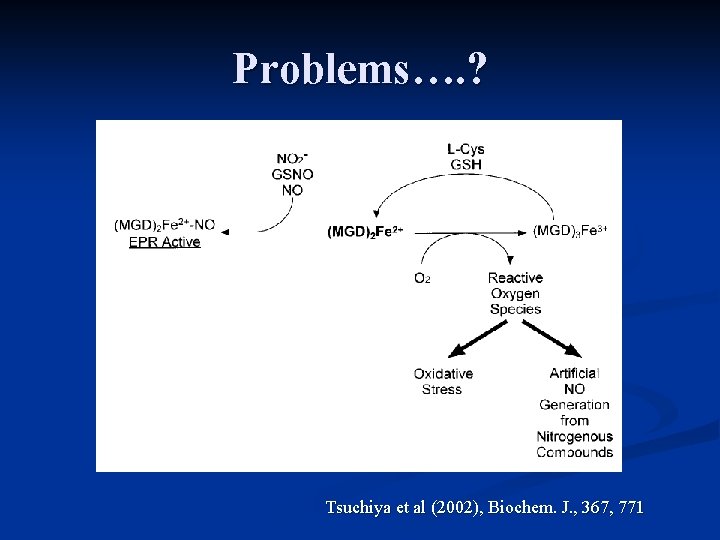
Problems…. ? Tsuchiya et al (2002), Biochem. J. , 367, 771

Iron/Dithiocarbamates Advantages/Disadvantages n n n Actually traps the NO – therefore 15 N experiments can be used to identify the source of the signal. Use in in vivo NO spin trapping and EPR imaging. Potential for signal from sources other than NO (Snitrosothiols/nitrite/HNO) Dithiocarbamates are good metal chelators and may inhibit metal ion-dependent enzymes (SOD, NOS etc). A Cu/dithiocarbamate signal overlaps the Fe/NO signal and can cause problems in situations where copper is present.

Hemoglobin/Myoglobin Reacts with NO with rate constant > 107 M-1 s-1 n Cheap and plentiful. n The reaction is accompanied by a UV-vis spectral change and a change in EPR spectrum n

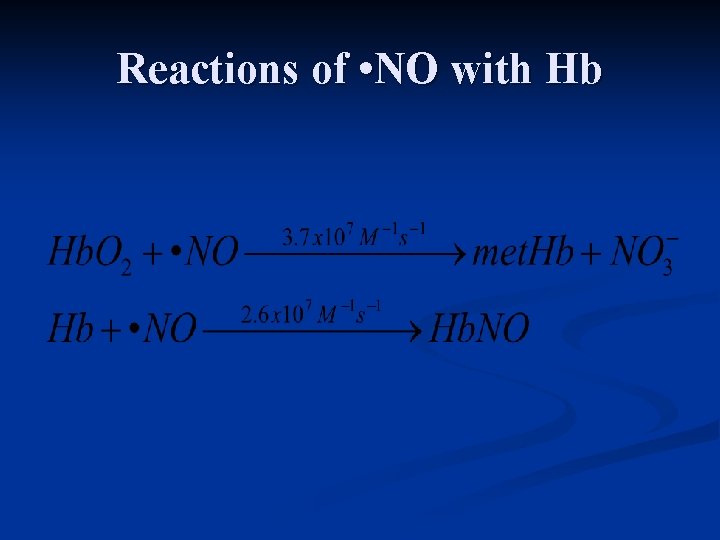
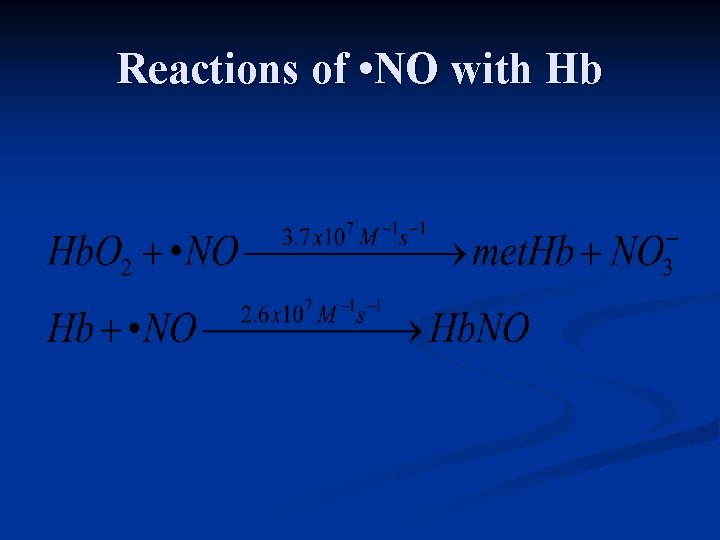
Reactions of • NO with Hb

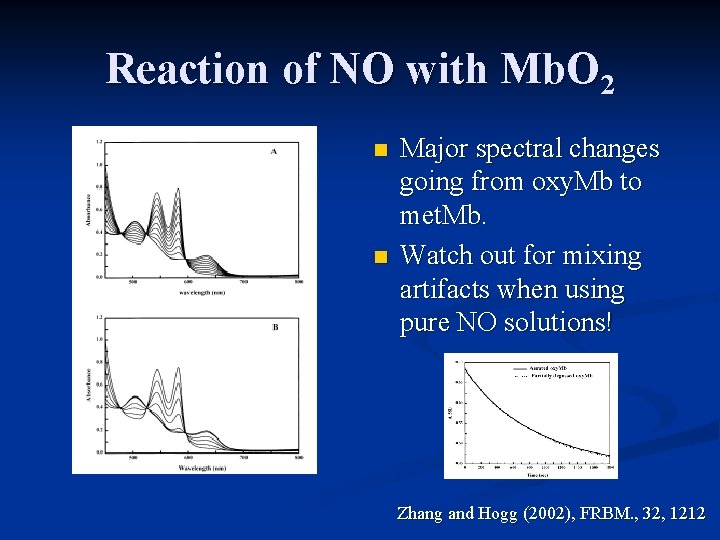
Reaction of NO with Mb. O 2 n n Major spectral changes going from oxy. Mb to met. Mb. Watch out for mixing artifacts when using pure NO solutions! Zhang and Hogg (2002), FRBM. , 32, 1212

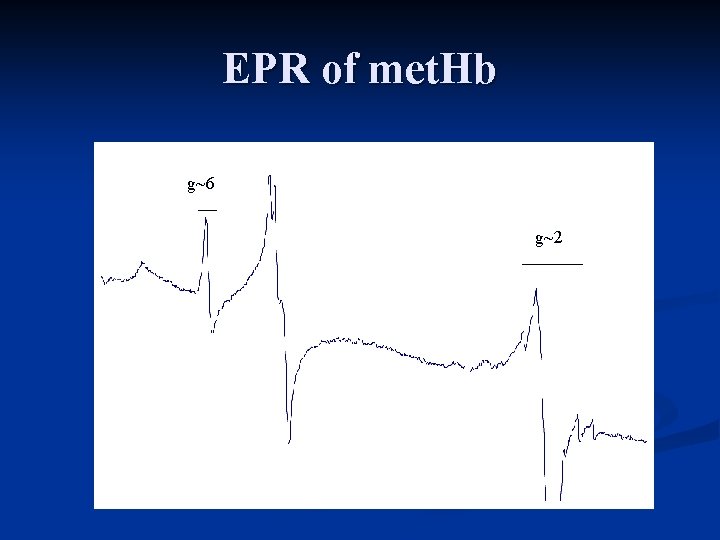
EPR of met. Hb g~6 g~2

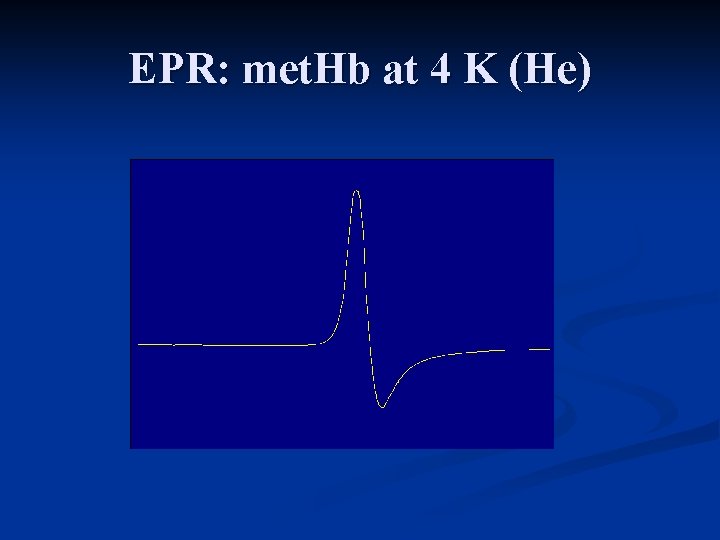
EPR: met. Hb at 4 K (He)

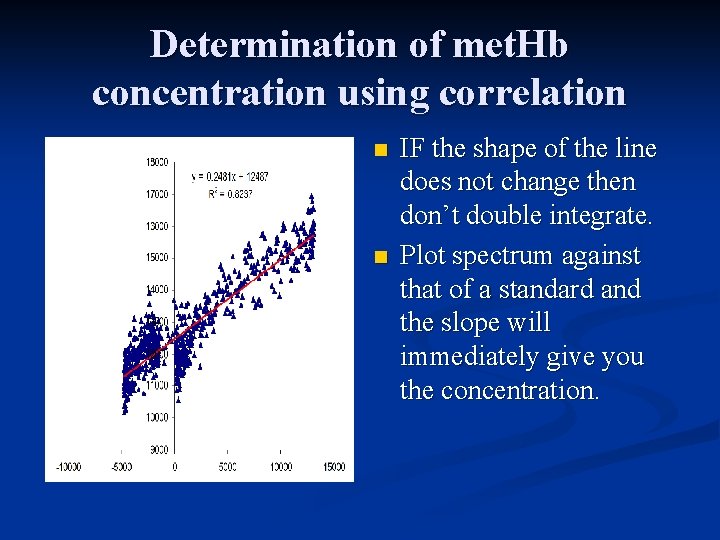
Determination of met. Hb concentration using correlation n n IF the shape of the line does not change then don’t double integrate. Plot spectrum against that of a standard and the slope will immediately give you the concentration.

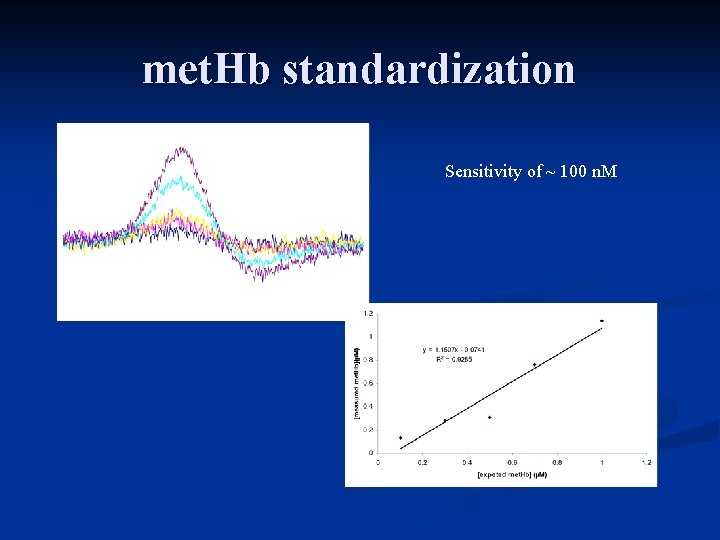
met. Hb standardization Sensitivity of ~ 100 n. M

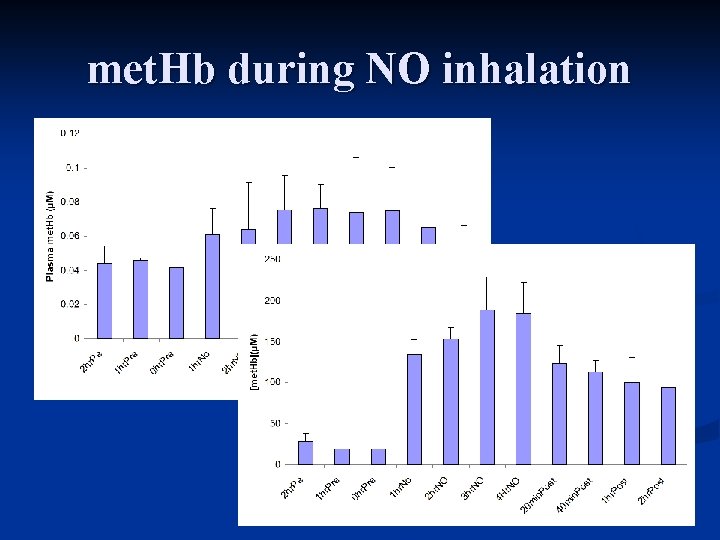
met. Hb during NO inhalation

Advantage/Disadvantages of met. Hb detection Simply easily analyzable signal. n Highly sensitive at liquid He temperatures n Not necessarily specific for NO (peroxynitrite and other oxidants could do the same thing) n NO is not ‘trapped’ and so cannot do 15 N experiments. n

Reactions of • NO with Hb

EPR: deoxy. Hb with NEM at 77 K N Fe 2+ NO

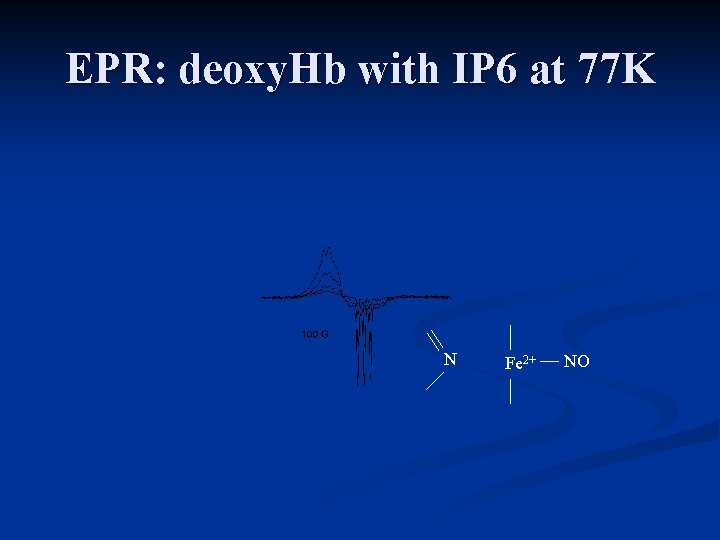
EPR: deoxy. Hb with IP 6 at 77 K N Fe 2+ NO

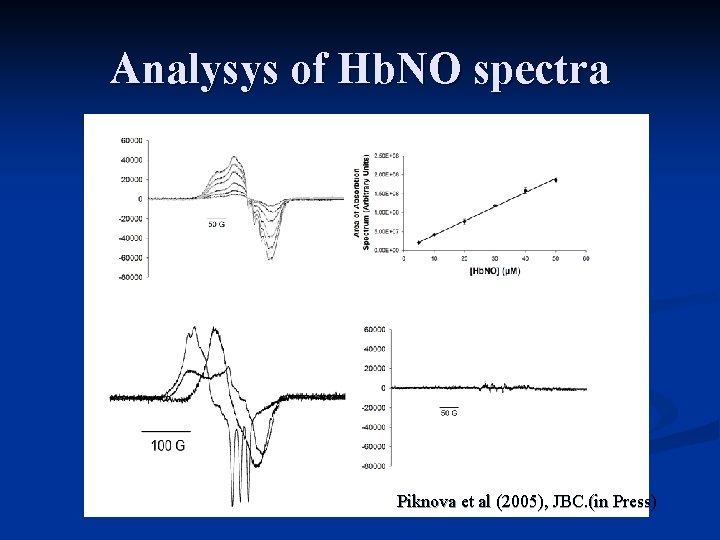
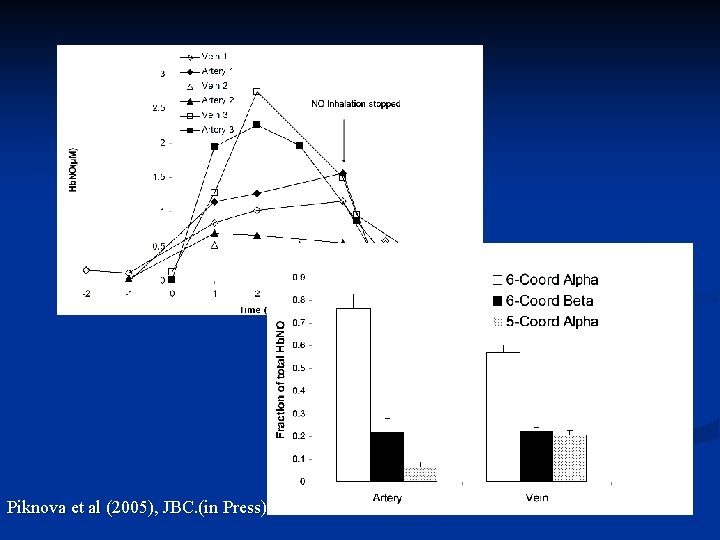
Analysys of Hb. NO spectra A C B i iii D ii Piknova et al (2005), JBC. (in Press)

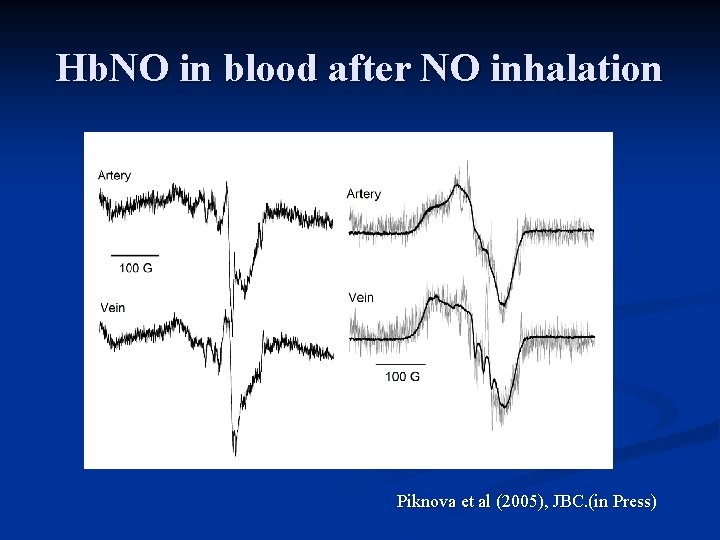
Hb. NO in blood after NO inhalation Piknova et al (2005), JBC. (in Press)

Piknova et al (2005), JBC. (in Press)

Advantage/Disadvantages of Hb. NO detection Complex multi-component signal. n Sensitive at liquid N 2 temperatures n NO is trapped and so can do 15 N experiments. n Needs to be deoxygenated!! n

In conclusion… n n EPR is a phenomenally useful tool in NO research for both in vitro, ex vivo and in vivo studies EPR direct detection of NO is possible after its stabilization by association with metal centers. EPR can also be detected by reactions that form or destroy paramagnetic species. Homework: Design a non-metallic, non-redox active NO spin-trap. Send compounds to Neil Hogg, Department of Biophysics, Medical College of Wisconsin, Milwaukee, WI.

Acknowledgements Barbora Piknova Yanhong Zhang Agnes Keszler Netanya Spencer Ravinder Singh National Biomedical EPR Center Medical College of Wisconsin (EB 001980) Raman Kalyanaraman Bill Antholine Brian Bennett Jim Hyde Mark Gladwin Alan Schechter Chris Reiter Dany Kim-Shapiro Ron Mason. . many others who’s work I have used
 Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Epoprotenol
Epoprotenol Bradykinin nitric oxide
Bradykinin nitric oxide Prime nitric oxide activator
Prime nitric oxide activator Nitric oxide properties
Nitric oxide properties Acid oxides examples
Acid oxides examples Magnesium reacting with nitric acid equation
Magnesium reacting with nitric acid equation Nitric acid and calcium carbonate
Nitric acid and calcium carbonate Calcium + nitric acid balanced equation
Calcium + nitric acid balanced equation Ionic equation of calcium carbonate and hydrochloric acid
Ionic equation of calcium carbonate and hydrochloric acid Nitric acid formal charge
Nitric acid formal charge Formal charge nco-
Formal charge nco- Nitric acid formal charge
Nitric acid formal charge Mechanical flow diagram
Mechanical flow diagram Nitric acid production plant
Nitric acid production plant Epr wwarszawa
Epr wwarszawa Targeted delivery
Targeted delivery Epr example
Epr example Whole airman concept epr bullets
Whole airman concept epr bullets Epr sue jennings
Epr sue jennings Epr cms
Epr cms Epr einstein podolsky rosen
Epr einstein podolsky rosen Epr
Epr Copenhagen interpretation
Copenhagen interpretation Epr.ulaanbaatar
Epr.ulaanbaatar Epr paradox
Epr paradox Sylvie richard edf
Sylvie richard edf Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worms-breton
Tư thế worms-breton Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Phép trừ bù
Phép trừ bù độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra