Effects of Estrogen and Nitric Oxide Deprivation on




- Slides: 13

Effects of Estrogen and Nitric Oxide Deprivation on Late-Developing Zebrafish Heart Health Presented by: Brice Scott, Nicole Blixt, Jeff Nutter, Ian Mc. Farland, Derrick Ziglar, and Garrett Parsons BI-420 W-01 Seminar Research Presentation Biology Department Virginia Military Institute 1

Introduction • Nitric oxide (NO) is a small gaseous molecule that has been found to be an intracellular messenger for major functions of the body such as blood flow, platelet aggregation, and heart and neural activity. • There are three nitric oxide synthase (NOS) isoforms: neuronal NOS (n. NOS), inducible NOS (i. NOS), and endothelial NOS (e. NOS). • Cardiac neuronal nitric oxide synthase (NOS 1 or n. NOS) has been studied in depth in regards to regulating myocardial contraction and relaxation. It is done so by targeting specific calcium (Ca 2+) handling proteins, protein kinase-dependent or phosphatase-dependent phosphorylation/dephosphorylation and redox homeostasis. 2

Introduction Cont. • The N-nitrosylation pathway has been implicated in protecting the heart from arrhythmic behavior. • Deficient S-nitrosylation of the cardiac ryanodine receptor, Ry. R 2, has a variable effect on the sarcoplasmic reticulum Ca 2+ leak in isolated myocytes, possibly causing heart arrhythmias. • DTT is an inhibitor of the N-nitrosylation pathway. 3

Hypotheses • Both Estrogen and nitric oxide deprivation can: – Cause similar significant anomalies in latedeveloping zebrafish heart rates. – Be quickly corrected through AI treatment and n. NOSI washout. • Arrhythmias in 4 -6 day old embryonic zebrafish can be caused by the inhibition of the S-nitrosylation pathway. 4

Methods • Zebrafish were kept in 28. 5 C incubator and the ERS control medium was changed out every 24 hours. • Fish used younger than five (5) days post-fertilization were exposed to protease for dechoronation. – Fish 5 -7 days post-fertilization were not exposed to protease • Heart rates were measured by a stopwatch up to 30 seconds then multiplying that number by 2 to obtain a value. • The “listless” condition was determined by the lack of swim escape function when stimulated with a probe. • Arrhythmic values were determined to be heart rates below 120 beats/min. 5

• AI study Methods Cont. – Treatments: ERS and 50 u. M of AI – Fish heart rates were evaluated and noted every 24 hours – After 48 hours, all fish were washed out with ERS • n. NOSI dose response study – Treatments: 75 u. M n. NOSI and ERS control group – Fish were monitored for 48 hours post-treatment for “listless” condition and heart rates – After 48 hours fish were washed out with ERS to determine the rate of recovery – HRs and “listless” condition recorded every 24 hours 6

Methods Cont. • DTT experiment – Treatment groups: DTT— 25 u. M, 50 u. M, 75 u. M and ERS as a control • DTT is an inhibitor of the N-nitrosylation pathway – Fish were monitored and evaluated after the first hour and then additionally every 24 hours, for a two day period. – Heart rates, the listless conditions, and survivability were marked. • Data analysis: – T-test, z-test, and ANOVA were used 7

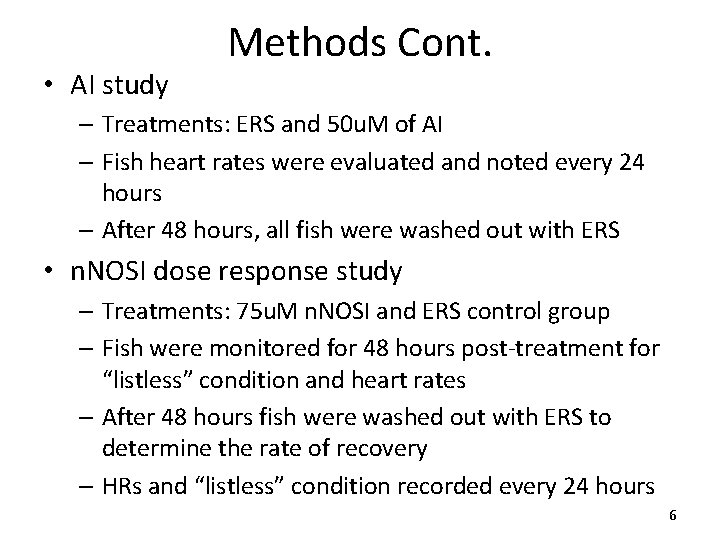
AI Experiment 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% Avg. Heart Rate Vs. Treatment 150 * 75. 0% ERS AI * 15. 2% Heart Rate BPM % Listless Percent (%) Listless Vs. Treatment 140 * 128. 8 130 * 106. 1 120 ERS AI 110 100 Control Treatment 50 μM Fig 1 - Illustrates the percentage of zebrafish population displaying the listless condition in treatments of ERS and AI. Note: The AI treatment causes a significant increase (*=p<0. 05) in the “listless” condition when compared with that of the control. Control 50 μM Treatment Fig 2 - Exhibits the zebrafish populations average heart rate when exposed to a dose mediated treatment of ERS or AI. Note: The AI treatment significantly reduces (*=p<0. 05) heart rates when compared with that of the controls. 8

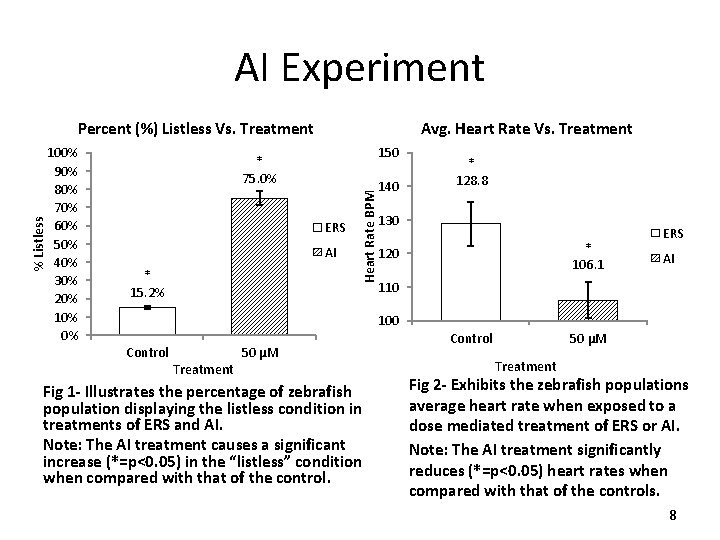
n. NOSI Experiment Percentage vs. Treatment Percentage (%) 120% 100% 80% 60% %survival 40% % listless 20% 0% ERS Control n. NOSi 75 μM Treatment Fig. 3 - Treatment at a concentration of 75 μM was analyzed after 48 hours beginning at 5 days post-fertilization. The percent survival and percent listless was calculated from all of the treatments. Note: n. NOSI treatment caused an increase in fish demonstrating the listless phenotype when compared to that of the ERS control group. 9

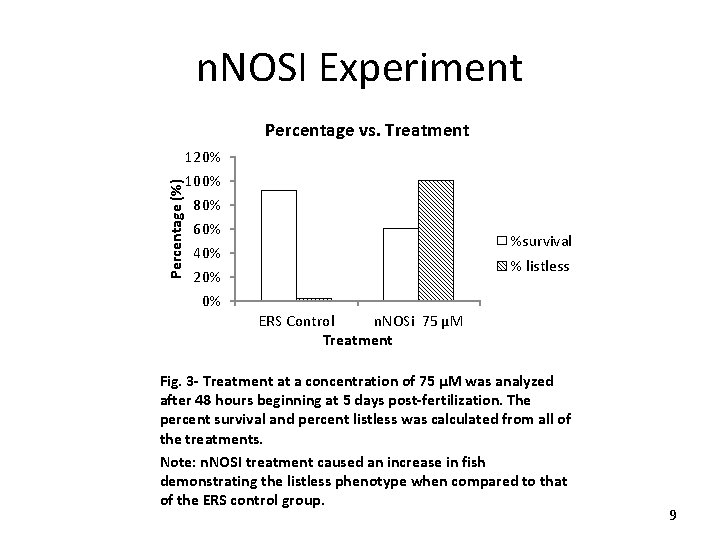
DTT Experiment Avg. Heart Rate Vs. Treatment Day 1 Heart Rate BPM 150 * 137. 5 140 133. 8 130 * 120. 3 120 ERS 110 DTT 100 Control 25 μM 50 μM Treatment 75 μM Fig. 4: Compiled dose-response data for day 1 of the heart rates (beats/min) in response to DTT treatment. Note: DTT treatment caused a significant decrease in heart rates at a 75 u. M concentration when compared to the control group (*=p<0. 05). 10

Conclusions • The data from the AI experiment demonstrated that: – AI treatment causes fish to display “listless” conditions within 75% of the population compared to 100% in the n. NOSI treatments. – A significant reduction in both heart rates and increased arrhythmias were observed similar to that of the n. NOSI treated fish. 11

Conclusions Cont. • The data from the n-NOSI dose-response and DTT experiment demonstrated: – DTT treatment significantly diminished heart rates and initiated greater arrhythmic values strongly indicating that the N-nitrosylation pathway is involved in this process. • This experiment also provided us with an ideal DTT dosage moving forward into the next phase of the experiment. 12

Help Received/Citations and Acknowledgments Help Received: Used information from Brice Scott’s research paper for introduction and COL Turner with revisions Citations: • Conti V, Russomanno G, Corbi G, Izzo V, Vecchione C, Filippelli A. 2013. Adrenoreceptors and nitric oxide in the cardiovascular system. Frontiers in Physiology. 4(321): 1 -11. • Cutler MJ, Plummer BN, Wan X, Sun QA, Hess D, Liu H, Deschenes I, Rosenbaum DS, Stamler JS, Laurita, KR. 2012. Aberrant s-nitrosylation mediates calcium-triggered ventricular arrhythmia in the intact heart. Proceedings of the National Academy of Sciences of the United States of America. 109(44): 18186 -18191. • Krause T, Gerbershagen MU, Fiege M, Weibhorn R, Wappler F. 2004. Dantrolene—a review of its pharmacology, therapeutic use and new developments. Anaesthesia. 59(4): 364 -373. • Zhang Y. 2013. Compartmentation of nitric oxide synthase regulates calcium signaling in the heart. Proceedings of the Physiology Society. 180 -181. • Zhao F, Li P, Chen SRW, Louis CF, Fruen BR. 2001. Dantrolene inhibition of ryanodine receptor Ca 2+ release channels: molecular mechanism and isoform sensitivity. The Journal of Biological Chemistry. 276: 13810 -13816. Acknowledgments: Thank you to the VMI Biology Department, COL Turner, Ms. Lozier, Cadet John Winalski ’ 16, and Cadet Vania Murcia ’ 17 for their help, critique, and advice this semester. 13
 Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Nitric oxide vs flolan
Nitric oxide vs flolan Catarrhal inflammation
Catarrhal inflammation Prime nitric oxide activator
Prime nitric oxide activator Nitric oxide properties
Nitric oxide properties Base oxides examples
Base oxides examples Answer key
Answer key Difference between estrogen and progesterone
Difference between estrogen and progesterone Layers of uterine wall
Layers of uterine wall Estrogen and gallstones
Estrogen and gallstones Fertilization and implantation
Fertilization and implantation Estrogen
Estrogen Estrogen progesterone
Estrogen progesterone Estrogen effect
Estrogen effect