The Search for Alternatives to Randomised Comparative Studies
























![Testicular Cancer Mortality in Germany, 19551999 [Becker and Boyle. Lancet. 1997; 350: 744] Testicular Cancer Mortality in Germany, 19551999 [Becker and Boyle. Lancet. 1997; 350: 744]](https://slidetodoc.com/presentation_image/d0b5822124f3c274bf3c4cc59d234fd4/image-30.jpg)





- Slides: 36

The Search for Alternatives to Randomised Comparative Studies Peter Boyle Division of Epidemiology and Biostatistics European Institute of Oncology Via Ripamonti 435 20141 Milan, Italy

Greater Participation • Governments and Non-Government Organisations are now increasingly mandating and urging a larger participation of patients in Clinical Trials. • For example, in England the National Cancer Research Network (NCRN) has been created and funded by the Government to increase the proportion of cancer patients in trials from 5% to 10% within five years.

More Obstacles and Higher Barriers • The number of administrative obstacles to establishing and conducting clinical trials is increasing. • There is an increasing burden in administering clinical trials. • An increasing number of oversights are in place with a corresponding increase in bureaucracy. • The situation could be described as having been poissoned.

Greater Expectation and Greater Reluctance • Patients have increasingly raised expectations of medical therapy. • They are increasingly reluctant to take part in trials where the choice of treatment is made by computer (like tossing a coin, according the BBC website two weeks ago). • Patients are increasingly seeking (untested) remedies from alternative medicine.

Ethics used to be a County beside Sussex • There is an increasing reluctance on the part of (lay) Ethics Committees (as well as patients) to accept the concept of randomisation. • There is also an increasing reluctance on the part of clinicians to participate in clinical trials given the increase in administrative bureaucracy. • Publishing Clinical Trials can now be a difficult process in view of the increasing stance taken by Medical Journal editors.

Clinical Trials • Randomised • Placebo controlled • Parallel group • Clinical Trial

The World Medical Association Declaration of Helsinki • Ethical Principles for Medical Research Involving Human Subjects. Adopted by the 18 th WMA General Assembly in Helsinki, Finland, June 1964 and amended by the • • • 29 th WMA General Assembly, Tokyo, Japan, October 1975 35 th WMA General Assembly, Venice, Italy, October 1983 41 st WMA General Assembly, Hong Kong, September, 1989 48 th WMA General Assembly, Somerset West, South Africa, 1996 52 nd WMA General Assembly, Edinburgh, Scotland, October 2000

The World Medical Association Declaration of Helsinki 1. The World Medical Association has developed the declaration of Helsinki as a statement of ethical principles to provide guidance to physicians and other participants in medical research involving human subjects. Medical Research involving human subjects includes research on identifiable human material and identifiable data. 4. Medical progress is based on research which ultimately must rest in part on experimentation involving human subjects.

The World Medical Association Declaration of Helsinki 10. It is the duty of the physician in medical research to protect the life, health, privacy and dignity of the human subject. 11. Medical research on human subjects must conform to generally accepted scientific principles, be based on a thorough knowledge of the scientific literature, other relevant sources of information, and on adequate laboratory and, where appropriate, animal experimentation.

The World Medical Association Declaration of Helsinki 10. It is the duty of the physician in medical research to protect the life, health, privacy and dignity of the human subject. 11. Medical research on human subjects must conform to generally accepted scientific principles, be based on a thorough knowledge of the scientific literature, other relevant sources of information, and on adequate laboratory and, where appropriate, animal experimentation.

Italian Randomised Trial of Tamoxifen • Randomised trial of intervention trial of tamoxifen in hysterectomised women began in 1992. • Tamoxifen declared a human carcinogen by the International Agency for Research on Cancer in 1994. • An Italian investigating Magistrate was informed of all this and sent his assistant to our Data Centre to obtain more details.

Italian Randomised Trial of Tamoxifen • Assistant magistrate arrived with three carabinieri armed with sub-machine guns. • Asked for names of women entered into the trial together with their address. • After discussing the ethics of confidentiality, how do you stop three armed men from obtaining such information from your computer? • Magistrate contacted these women and asked several questions including whether they had been asked to pay money to enter the trial.

Italian Randomised Trial of Tamoxifen Medical Research Under Dictatorship

The World Medical Association Declaration of Helsinki 19. Medical research is only justified if there is a reasonable likelihood that the populations in which the research is carried out stand to benefit from the results of the research. 29. The benefits, risks, burdens, and effectiveness of a new method should be tested against those of the best current prophylactic, diagnostic, and therapeutic methods. This does not exclude the use of placebo, or no treatment, in studies where no proven prophylactic, diagnostic or therapeutic method exists.

The World Medical Association Declaration of Helsinki • To further clarify the WMA position on the use of placebo controlled trials, the WMA Council issued during October, 2001, a note of clarification on article 29.

The World Medical Association Declaration of Helsinki The WMA is concerned that paragraph 29 of the revised Declaration of Helsinki (October, 2000) has led to diverse interpretations and possible confusion. It hereby reaffirms its position that extreme care must be taken in use of the placebo-controlled trial and that in general this methodology should only be used in the absence of existing proven therapy. However, a placebocontrolled trial may be ethically acceptable, even if proven therapy is available, under the following circumstances: Where for compelling and scientifically sound methodological reasons its use is necessary to determine the efficacy or safety of a prophylactic, diagnostic or therapeutic method; or Where a prophylactic, diagnostic or therapeutic method is being investigated for a minor condition and the patients who receive placebo will not be subject to any additional risk of serious or irreversible harm. All other provisions of the Declaration of Helsinki must be adhered to, especially the need for appropriate ethical and scientific review.

The Threat to Placebo-Controlled Trials • The threat to placebo controlled trials, the most visible being from the revision of the Declaration of Helsinki, is a major handicap in many fields of Medicine where there is no established and agreed standard of care. • There are major risks to areas such as benign urological conditions, such as Benign Prostatic Hyperplasia (BPH), Urinary Incontinence, Prostatitis and Erectile Dysfunction. • Although benign in terms of life threatening, these conditions can be malignant in terms of the impact on quality of life. And they are common: it has been shown that six out of every seven couples over the age of 70 have at least one such condition

Conduct of Studies for Regulatory Purposes • With developments in the European Medical Evaluation Agency there is no move to take any ethnic-racial issues into consideration when approving drugs in Europe, in the sense that there is no requirement to show separately efficacy and adverse events in Nordic populations, Slavic Populations, Mediterranean populations or Latin populations. • Results of studies conducted in North America are equally accepted in Europe: the only issue is the quality of the studies.

Benign Urological Conditions • Drug therapy is now available for many of these conditions and quite useful, particularly in BPH. However, it is not effective in severe disease when surgery is much more effective at reducing symptoms and increasing urinary flow rates. • If we stopped placebo controlled trials and insisted on a standard-of-care, then surgery would be the natural standard: no pharmacological therapy approaches the efficacy of surgery. • But many men with mild to moderate symptoms, who get major improvements in quality of life from medical therapy, could eventually be denied that therapy and have to wait until their symptoms are severe enough to justify surgery.

Some Important Issues for the Future • There has always been a major puzzle about why some patients respond to particular therapy while in almost identical circumstances, others do not. • With increasing insights into molecular genetics parts of this puzzle are about to become clear.

Genetically Targetted Treatments • Herceptin® (Trastuzumab) is used to treat HER 2 -driven metastatic breast cancer with some outstanding responses. • Gleevec® (imatinib mesylate) is indicated for the treatment of patients with Philadelphia chromosome positive (Ph+) chronic myeloid leukaemia (CML) in blast crisis, accelerated phase, or in chronic phase after failure of interferon-alpha therapy. • These are pioneering breakthroughs and will soon be followed by more in a similar vein. Specific molecules being developed to attack particular molecular or genetic targets.

Food for thought. . . • Major home-run treatments for cancer have generally not proceeded to Phase III trials (platinum, MOPP) and now Gleevec (where accelerated approval was based on time to progression as the primary surrogate endpoint). • Many of these treatments need to be delivered in specialist treatment centres and they are frequently expensive. • Availability of curative therapy is still a real issue in the 21 st Century.

Testicular Cancer “ It is widely appreciated that the application of chemotherapy in the treatment of germ cell tumours exemplifies the best results to be expected from this approach to solid tumours, since the majority of patients treated are now cured. ” Kaye SB and Boyle P Cancer Surveys 1989; 8: 632 -646

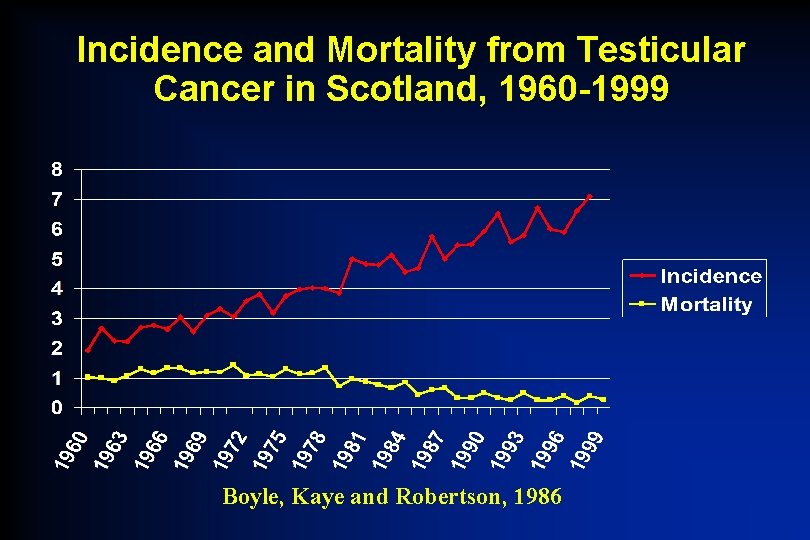
Incidence and Mortality from Testicular Cancer in Scotland, 1960 -1999 Boyle, Kaye and Robertson, 1986

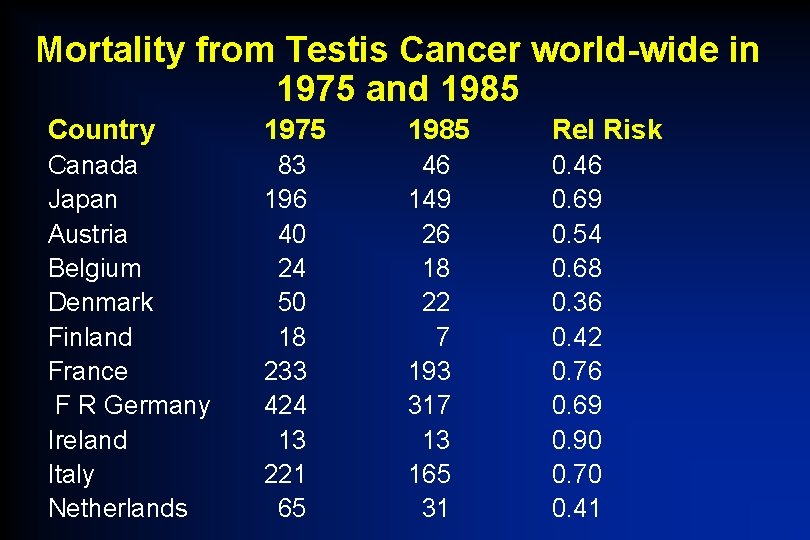
Mortality from Testis Cancer world-wide in 1975 and 1985 Country 1975 1985 Rel Risk Canada Japan Austria Belgium Denmark Finland France F R Germany Ireland Italy Netherlands 83 196 40 24 50 18 233 424 13 221 65 46 149 26 18 22 7 193 317 13 165 31 0. 46 0. 69 0. 54 0. 68 0. 36 0. 42 0. 76 0. 69 0. 90 0. 70 0. 41

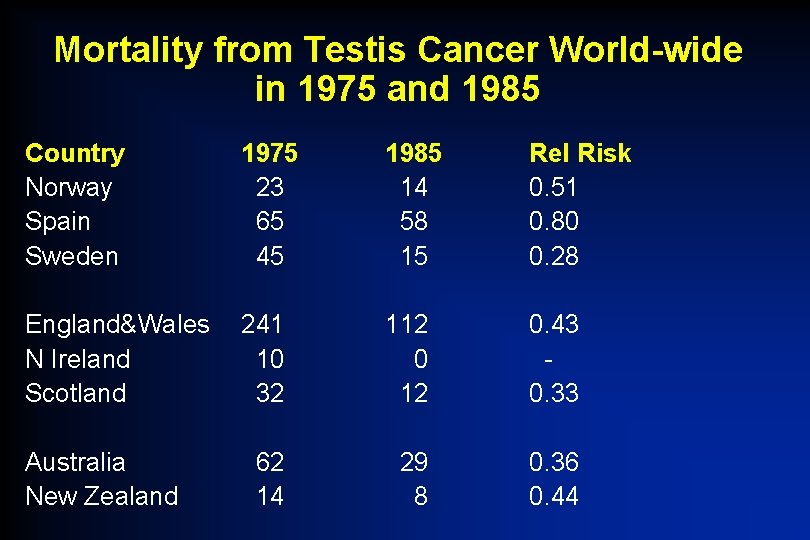
Mortality from Testis Cancer World-wide in 1975 and 1985 Country Norway Spain Sweden 1975 23 65 45 1985 14 58 15 Rel Risk 0. 51 0. 80 0. 28 England&Wales N Ireland Scotland 241 10 32 112 0. 43 0. 33 62 14 29 8 0. 36 0. 44 Australia New Zealand

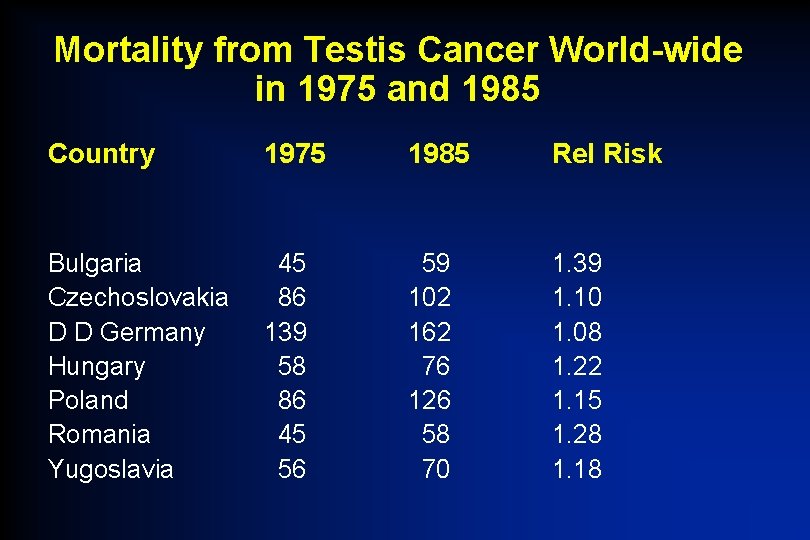
Mortality from Testis Cancer World-wide in 1975 and 1985 Country 1975 1985 Rel Risk Bulgaria Czechoslovakia D D Germany Hungary Poland Romania Yugoslavia 45 86 139 58 86 45 56 59 102 162 76 126 58 70 1. 39 1. 10 1. 08 1. 22 1. 15 1. 28 1. 18

“ 80 -90% of patients with testicular cancer can now expect to be cured of their disease and in most countries this seems to be so, but not in Central and Eastern Europe where about 1 in 2 cases may die of their disease. Any fundamental difference in biological behaviour is unlikely and a more likely explanation is that the differences in mortality relate to delivery of curative chemotherapy, including cisplatin, or to deficiencies in patterns of referral. ” Boyle P, Maisonneuve P and Kaye SB Lancet 1990; 335: 1033 -34

Testicular Cancer Mortality in Germany, 1955 -1995 • The economic situation in central and eastern European countries underwent radical change together with major changes in health care. • Arguably, the most sudden and dramatic change took place in the DDR (Eastern Germany).
![Testicular Cancer Mortality in Germany 19551999 Becker and Boyle Lancet 1997 350 744 Testicular Cancer Mortality in Germany, 19551999 [Becker and Boyle. Lancet. 1997; 350: 744]](https://slidetodoc.com/presentation_image/d0b5822124f3c274bf3c4cc59d234fd4/image-30.jpg)
Testicular Cancer Mortality in Germany, 19551999 [Becker and Boyle. Lancet. 1997; 350: 744]

Testicular Cancer Mortality in Germany, 1955 -1995 “The decline in mortality from testicular cancer in the former DDR has paralleled the economic changes after German unification and lends support to the hypothesis that economic factors had previously limited the implementation of new treatment for this curable cancer. ” Becker N and Boyle P Lancet 1997; 350: 744

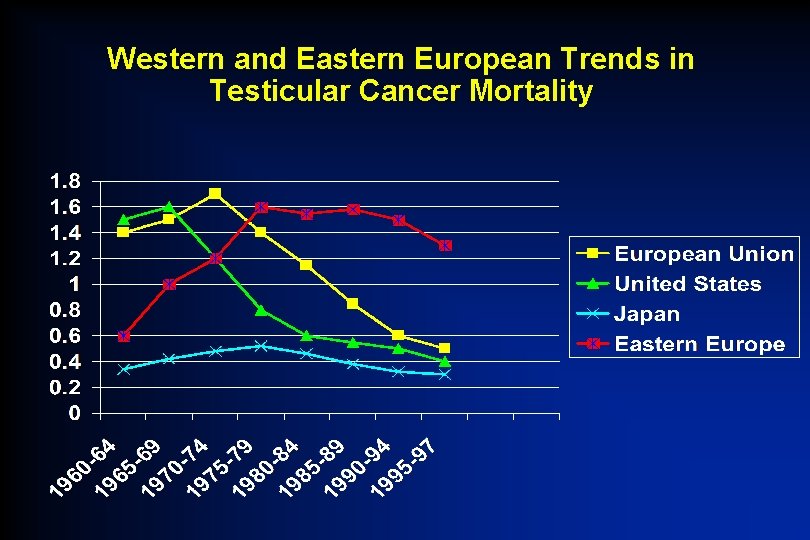
Western and Eastern European Trends in Testicular Cancer Mortality • To see how the situation has been evolving, we went back to the most recent data on testicular cancer mortality in Eastern Europe, Western Europe, the United States and Japan. Levi F, La Vecchia C, Boyle P Lancet 2001; 357: 1853 -4

Western and Eastern European Trends in Testicular Cancer Mortality

Western and Eastern European Trends in Testicular Cancer Mortality “Our results indicate, however, that in Central and Eastern Europe, widespread and substantial inadequacies exist in adopting adequate treatments. This situation needs to be remedied. ” Levi F, La Vecchia C, Boyle P Lancet 2001; 357: 1853 -4

Shaping the Future • Even common drugs used in common diseases can have great variation in their outcomes in individual patients and genetic explanations of such differences are starting to be sought. • It is possible, particularly in a disease such as cancer where standards of care have been established for many diseases, that in coming years the randomised trial will be outdated and replaced (perhaps) by the creation of a large clinical and biological database with diagnostic, treatment, genetic and response information available. • A new drug could quickly be tested for (superior) efficacy against similar patients with similar tumours treated in the same database and matched for key factors including genetic mutations and alterations.

Summary and Conclusions • There will be many changes in the evaluation of new molecules in the treatment of human disease in the coming years. Our increasing knowledge in areas such as molecular genetics will be brought to bear on how and in what circumstances new remedies work. • What was futuristic only a few years ago is becoming increasingly the reality. The best way to shape the future is to start planning how it should look today. • Meetings such as these facing up to current reality could prove very useful in determing how we go forward and what the time frame for change will be. • It is essential that there be a partnership formed with Regulatory Agencies, Pharmaceutical Industry and external (independent) scientists to determine how to proceed.