Stoichiometry Chemistry Chapter 9 Introduction to Stoichiometry Composition











- Slides: 20

Stoichiometry Chemistry Chapter 9

Introduction to Stoichiometry • Composition Stoichiometry…Chapter 3 dealt with the mass relationships of elements in compounds. • Reaction Stoichiometry…this chapter…involves the mass relationships between reactants and products in a chemical reaction.

Introduction • ALL stoichiometry problems begin with A BALANCED EQUATION! • Problems are classified according to the information given and the information you are expected to find (the unknown).

FOUR TYPES OF PROBLEMS • Type 1: Given and Unknown quantities are amounts in moles. – Amt. given (in mole) >>>>>amt. unknown (in mol) • Type 2: Given is an amount in moles and the unknown is a mass expressed in grams. – Amt. given (in mol) >>> amt. unknown (in mol)>>> mass of unknown • Type 3: Given is a mass in grams and the unknown is an amount in moles. – Mass given (in g)>>>amt. given (in mol)>>>amt. unknown (in mol) • Type 4: Given is a mass in grams and the unknown is a mass in grams. – Amt. give (in g)>>>amt. given (mol)>>>amt. unknown(mol)>>>amt. unkn(in g)

MOLE TO MOLE • You must make comparisons/conversions between different substance using MOLES. – We call this: using mole ratios. A mole ratio is a conversion factor that relates the amounts in moles of any two substances involved in a chemical reaction. • GET THIS INFO FROM THE BALANCED EQUATION!

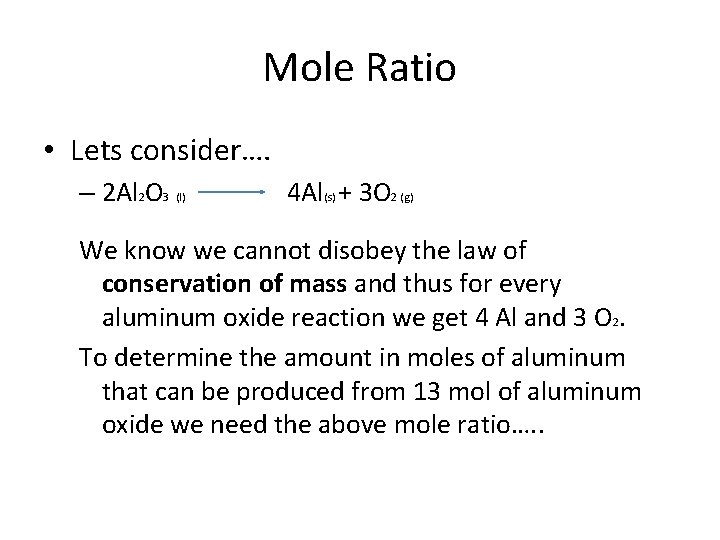
Mole Ratio • Lets consider…. – 2 Al 2 O 3 (l) 4 Al(s) + 3 O 2 (g) We know we cannot disobey the law of conservation of mass and thus for every aluminum oxide reaction we get 4 Al and 3 O 2. To determine the amount in moles of aluminum that can be produced from 13 mol of aluminum oxide we need the above mole ratio…. .

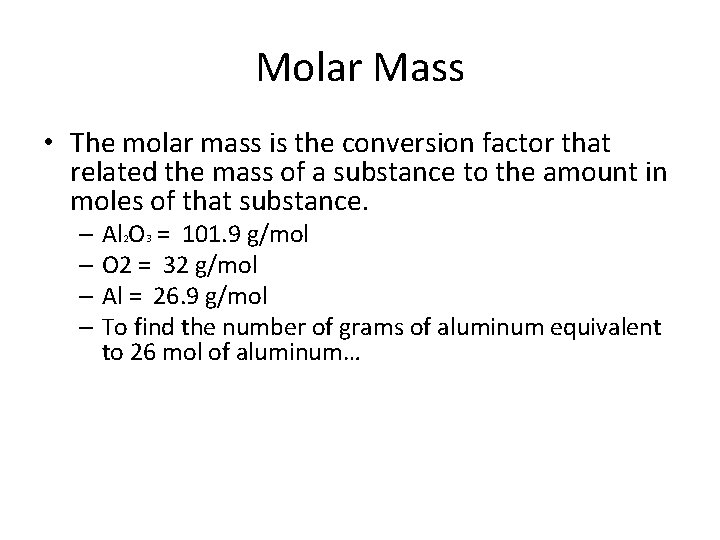
Molar Mass • The molar mass is the conversion factor that related the mass of a substance to the amount in moles of that substance. – Al 2 O 3 = 101. 9 g/mol – O 2 = 32 g/mol – Al = 26. 9 g/mol – To find the number of grams of aluminum equivalent to 26 mol of aluminum…

IDEAL stocihiometric calcuations We are working with predictions about chemical reactions and theoretical stoichiometeric calculations. In other words we are dealing with IDEAL conditions (which rarely occur in the lab. ). Working stoichiometeric calculations requires a logical, systematic approach to problem solving. ONLY PRACTICE can make it easy and understandable.

NEW MOLE TUNNEL PROBLEMS • See worksheet!!! – Step one: Balance the equation – Step Two: Determine the formula weights of the compounds. – Step Three: Determine what we “have” and what we “want. ” – Step Four: Use the mole tunnel to move from haves to wants….

DRAW THE NEW MOLE TUNNEL

Let’s practice: from worksheet • 90 Grams of hydrogen will produce how many grams of iron? Fe O + H Fe + H O Step 1: (balance equation) 3 4 2 2 Step 2: (determine formula weights) Step 3: (determine have and wants & where to start on mole tunnel) Step 4: ( work with new mole tunnel to solve)

Limiting Reagents and Percent Yield • In the laboratory it is rare that a reaction is carried out with exactly the required amounts of all reactants. • Usually we have excess of one compound. • The reactant that is used up first is called the ____________. – This reagent limits the amount of product that can be made because it limits the amount of reactants that can combine.

Limiting Reagents • Think of finding a date for a dance…. if there are only 47 boys and 58 girls…. then only 47 of the girls will have dates. – The number of boys is the limiting factor (limiting reagent). They limit the amount of couples at the dance. – This reasoning can also apply to chemical reactions.

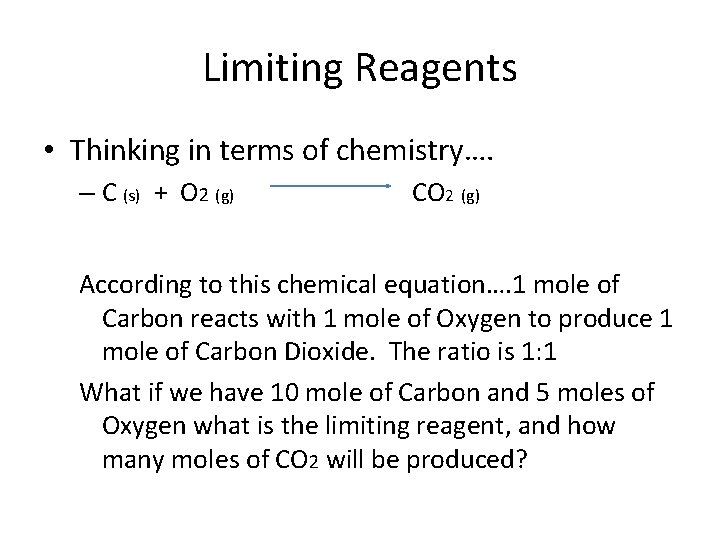
Limiting Reagents • Thinking in terms of chemistry…. – C (s) + O 2 (g) CO 2 (g) According to this chemical equation…. 1 mole of Carbon reacts with 1 mole of Oxygen to produce 1 mole of Carbon Dioxide. The ratio is 1: 1 What if we have 10 mole of Carbon and 5 moles of Oxygen what is the limiting reagent, and how many moles of CO 2 will be produced?

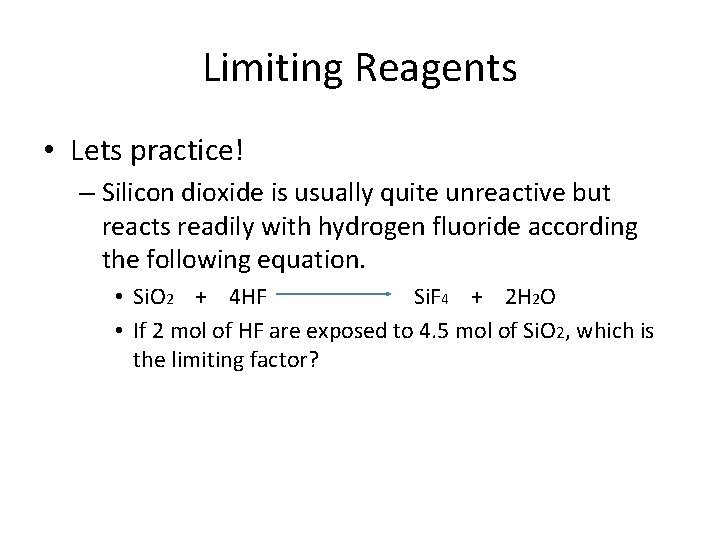
Limiting Reagents • Lets practice! – Silicon dioxide is usually quite unreactive but reacts readily with hydrogen fluoride according the following equation. • Si. O 2 + 4 HF Si. F 4 + 2 H 2 O • If 2 mol of HF are exposed to 4. 5 mol of Si. O 2, which is the limiting factor?

• Work this much like a NEW mole tunnel (stoichiometry problem). – Balance the equation and use the mole ratios to solve.

More Practice on limiting reagents • Some rocket engines use a mixture of hydrazine, N 2 H 4, and hydrogen peroxide, H 2 O 2, as the propellant. – N 2 H 2 + H 2 O 2 N 2 + H 2 O – Which is the limiting reagent, when 0. 75 mol of N 2 H 2 is mixed with 0. 50 mols of H 2 O 2?

Percent Yield • The amounts of products we have calculated in our stoichiometric problems so far represent theoretical yields. – Theoretical yields are the maximum amounts of product that can be produced from a given amount of reactants. – Generally in the lab. We obtain less than theoretical amounts…. . competing side reactions, loss during purification, experimental error, etc. – The measure amount of product obtained from a reaction is called __________ of that product.

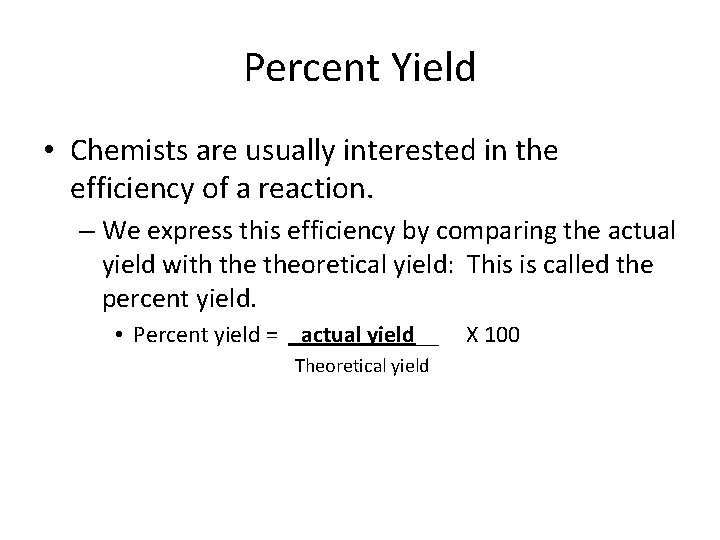
Percent Yield • Chemists are usually interested in the efficiency of a reaction. – We express this efficiency by comparing the actual yield with theoretical yield: This is called the percent yield. • Percent yield = _actual yield__ Theoretical yield X 100

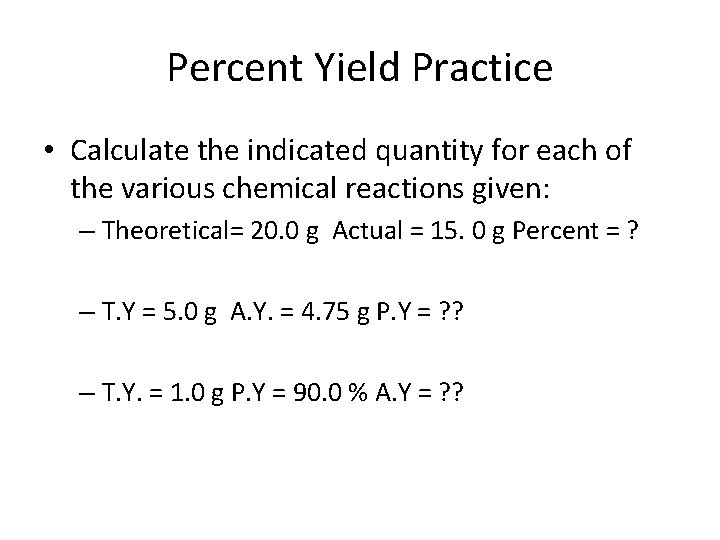
Percent Yield Practice • Calculate the indicated quantity for each of the various chemical reactions given: – Theoretical= 20. 0 g Actual = 15. 0 g Percent = ? – T. Y = 5. 0 g A. Y. = 4. 75 g P. Y = ? ? – T. Y. = 1. 0 g P. Y = 90. 0 % A. Y = ? ?