Short Version 19 2 nd Law of Thermodynamics



- Slides: 26

Short Version : 短版: 19. 2 nd Law of Thermodynamics 19. 熱力學第二定律

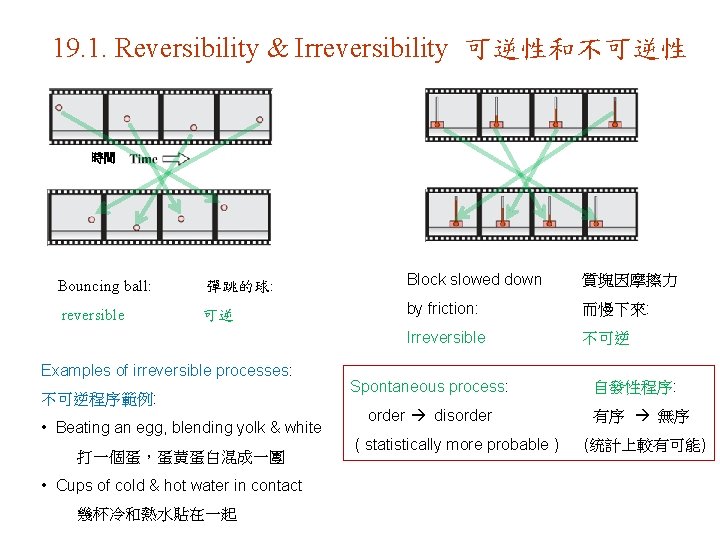
19. 1. Reversibility & Irreversibility 可逆性和不可逆性 時間 Bouncing ball: 彈跳的球: Block slowed down 質塊因摩擦力 reversible 可逆 by friction: 而慢下來: Irreversible 不可逆 Examples of irreversible processes: 不可逆程序範例: • Beating an egg, blending yolk & white 打一個蛋,蛋黄蛋白混成一團 • Cups of cold & hot water in contact 幾杯冷和熱水貼在一起 Spontaneous process: order disorder ( statistically more probable ) 自發性程序: 有序 無序 (统計上較有可能)

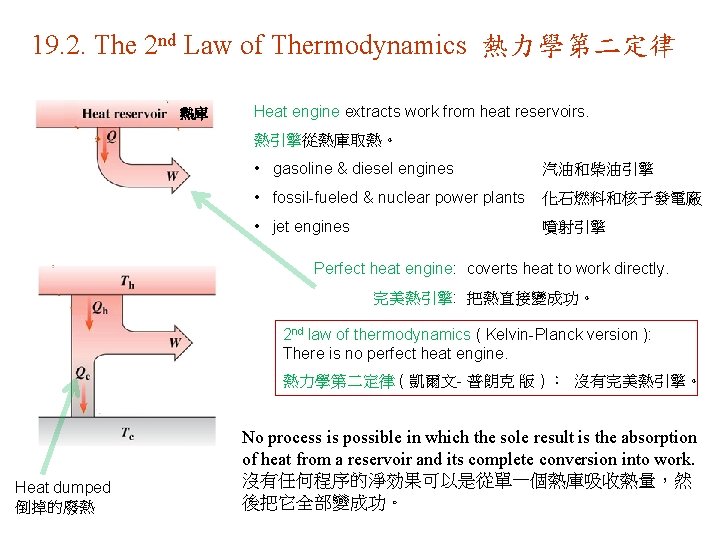
19. 2. The 2 nd Law of Thermodynamics 熱力學第二定律 熱庫 Heat engine extracts work from heat reservoirs. 熱引擎從熱庫取熱。 • gasoline & diesel engines 汽油和柴油引擎 • fossil-fueled & nuclear power plants 化石燃料和核子發電廠 • jet engines 噴射引擎 Perfect heat engine: coverts heat to work directly. 完美熱引擎: 把熱直接變成功。 2 nd law of thermodynamics ( Kelvin-Planck version ): There is no perfect heat engine. 熱力學第二定律 ( 凱爾文- 普朗克 版 ) : 沒有完美熱引擎。 Heat dumped 倒掉的廢熱 No process is possible in which the sole result is the absorption of heat from a reservoir and its complete conversion into work. 沒有任何程序的淨効果可以是從單一個熱庫吸收熱量,然 後把它全部變成功。

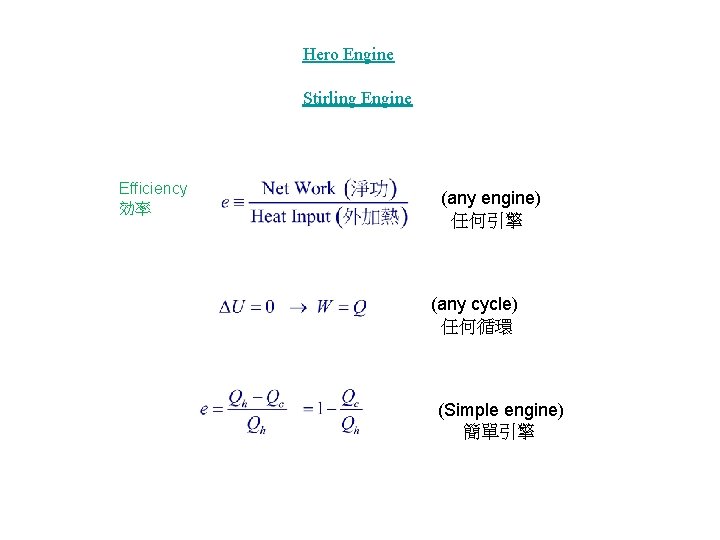
Hero Engine Stirling Engine Efficiency 効率 (any engine) 任何引擎 (any cycle) 任何循環 (Simple engine) 簡單引擎

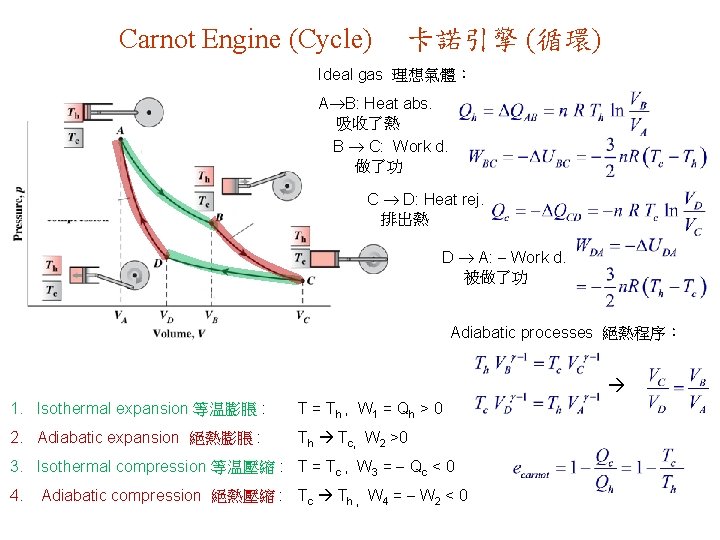
Carnot Engine (Cycle) 卡諾引擎 (循環) Ideal gas 理想氣體: A B: Heat abs. 吸收了熱 B C: Work d. 做了功 C D: Heat rej. 排出熱 D A: Work d. 被做了功 Adiabatic processes 絕熱程序: 1. Isothermal expansion 等温膨脹 : T = Th , W 1 = Q h > 0 2. Adiabatic expansion 絕熱膨脹 : Th Tc, W 2 >0 3. Isothermal compression 等温壓縮 : T = Tc , W 3 = Qc < 0 4. Adiabatic compression 絕熱壓縮 : Tc Th , W 4 = W 2 < 0

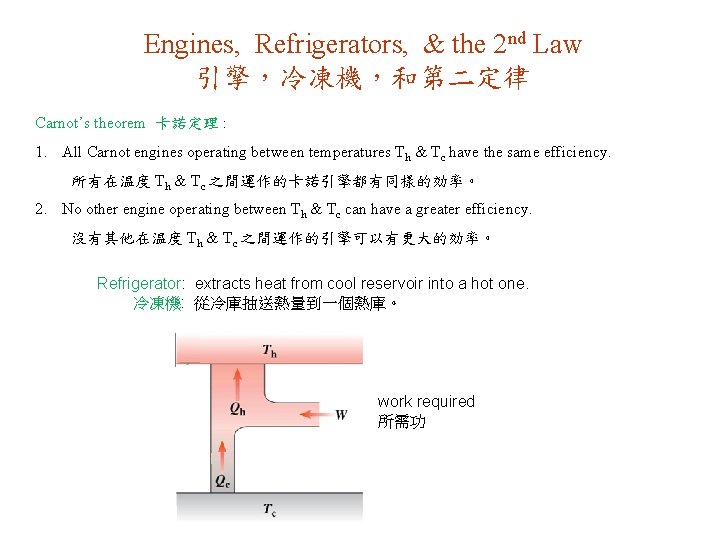
Engines, Refrigerators, & the 2 nd Law 引擎,冷凍機,和第二定律 Carnot’s theorem 卡諾定理 : 1. All Carnot engines operating between temperatures Th & Tc have the same efficiency. 所有在温度 Th & Tc 之間運作的卡諾引擎都有同樣的効率。 2. No other engine operating between Th & Tc can have a greater efficiency. 沒有其他在温度 Th & Tc 之間運作的引擎可以有更大的効率。 Refrigerator: extracts heat from cool reservoir into a hot one. 冷凍機: 從冷庫抽送熱量到一個熱庫。 work required 所需功

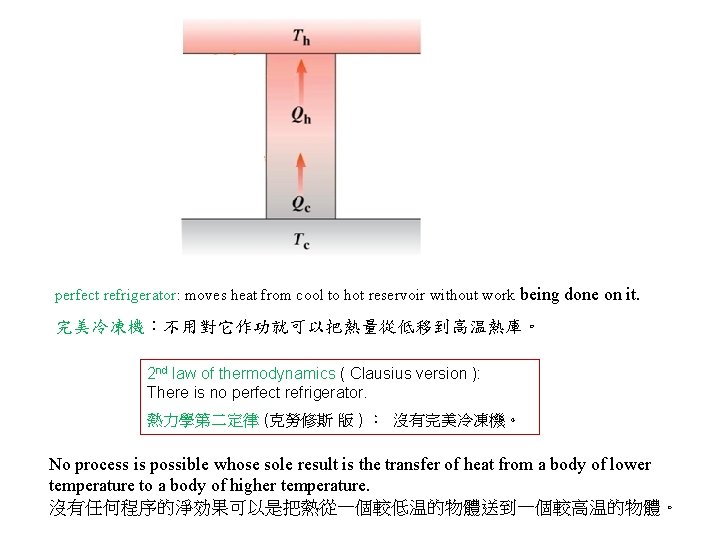
perfect refrigerator: moves heat from cool to hot reservoir without work being done on it. 完美冷凍機:不用對它作功就可以把熱量從低移到高温熱庫。 2 nd law of thermodynamics ( Clausius version ): There is no perfect refrigerator. 熱力學第二定律 (克勞修斯 版 ) : 沒有完美冷凍機。 No process is possible whose sole result is the transfer of heat from a body of lower temperature to a body of higher temperature. 沒有任何程序的淨効果可以是把熱從一個較低温的物體送到一個較高温的物體。

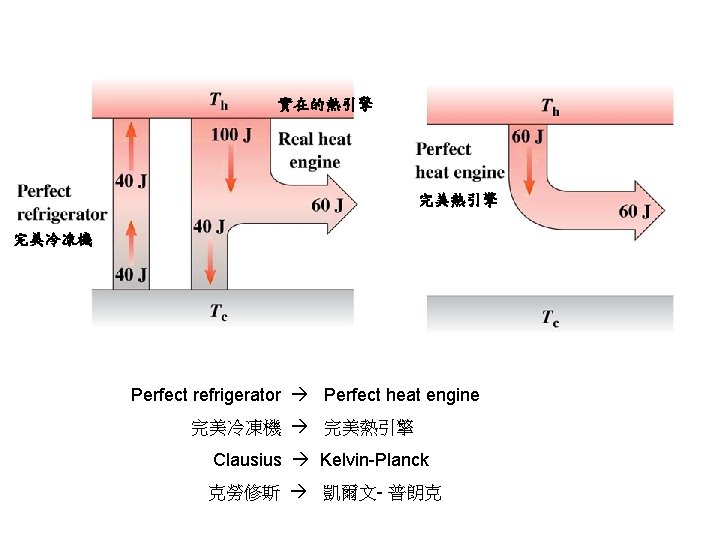
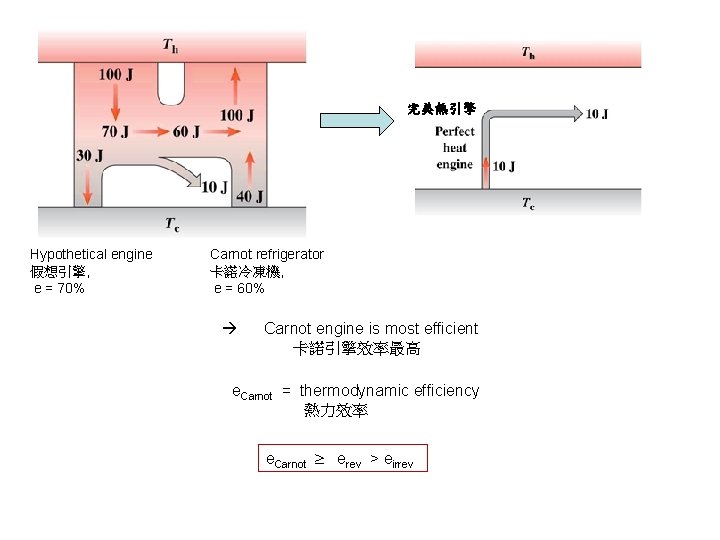
實在的熱引擎 完美冷凍機 Perfect refrigerator Perfect heat engine 完美冷凍機 完美熱引擎 Clausius Kelvin-Planck 克勞修斯 凱爾文- 普朗克

完美熱引擎 Hypothetical engine 假想引擎, e = 70% Carnot refrigerator 卡諾冷凍機, e = 60% Carnot engine is most efficient 卡諾引擎效率最高 e. Carnot = thermodynamic efficiency 熱力效率 e. Carnot erev > eirrev

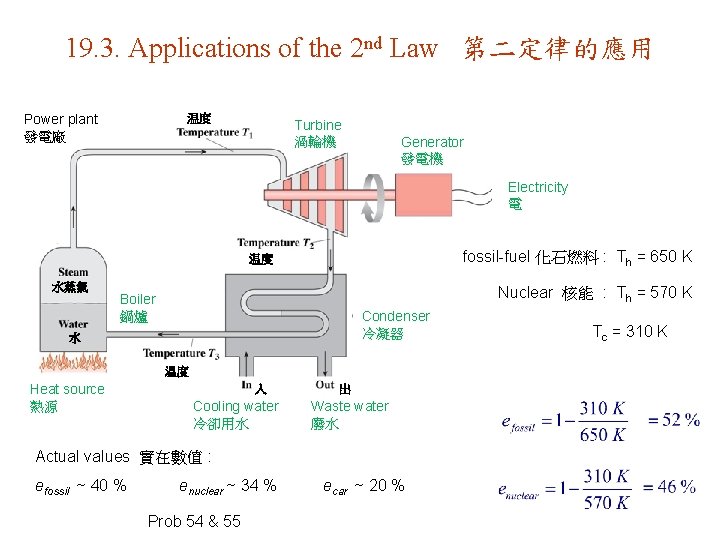
19. 3. Applications of the 2 nd Law 第二定律的應用 Power plant 發電廠 温度 Turbine 渦輪機 Generator 發電機 Electricity 電 fossil-fuel 化石燃料 : Th = 650 K 温度 水蒸氣 Nuclear 核能 : Th = 570 K Boiler 鍋爐 Condenser 冷凝器 水 温度 Heat source 熱源 入 Cooling water 冷卻用水 出 Waste water 廢水 Actual values 實在數值 : efossil ~ 40 % enuclear ~ 34 % Prob 54 & 55 ecar ~ 20 % Tc = 310 K

Application: Combined-Cycle Power Plant 應用: 聯合-循環發電廠 Turbine engines: high Th ( 1000 K 2000 K ) & Tc ( 800 K ) … not efficient. 渦輪機: 高 Th ( 1000 K 2000 K ) & Tc ( 800 K ) … 無効率。 Steam engines : Tc ~ ambient 300 K. 蒸氣機 : Tc ~ 周圍的 300 K. Combined-cycle 聯合-循環 : Th ( 1000 K 2000 K ) & Tc ( 300 K ) … e ~ 60%

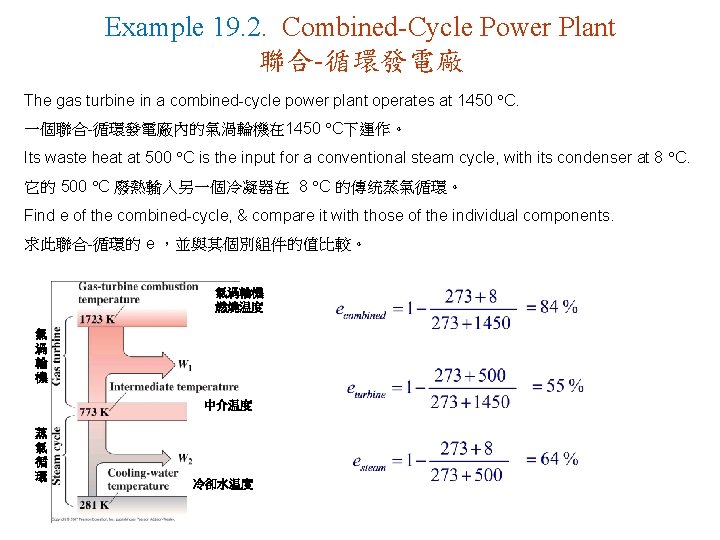
Example 19. 2. Combined-Cycle Power Plant 聯合-循環發電廠 The gas turbine in a combined-cycle power plant operates at 1450 C. 一個聯合-循環發電廠內的氣渦輪機在 1450 C下運作。 Its waste heat at 500 C is the input for a conventional steam cycle, with its condenser at 8 C. 它的 500 C 廢熱輸入另一個冷凝器在 8 C 的傳统蒸氣循環。 Find e of the combined-cycle, & compare it with those of the individual components. 求此聯合-循環的 e ,並與其個別組件的值比較。 氣渦輪機 燃燒温度 氣 渦 輪 機 中介温度 蒸 氣 循 環 冷卻水温度

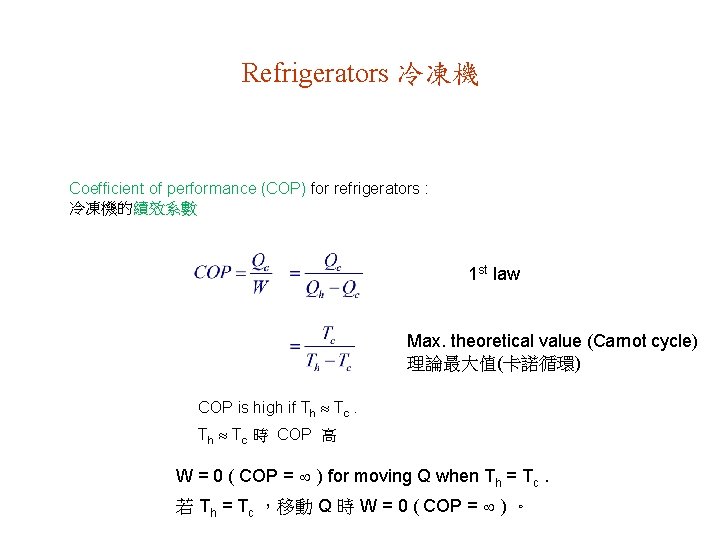
Refrigerators 冷凍機 Coefficient of performance (COP) for refrigerators : 冷凍機的績效系數 1 st law Max. theoretical value (Carnot cycle) 理論最大值(卡諾循環) COP is high if Th Tc 時 COP 高 W = 0 ( COP = ) for moving Q when Th = Tc. 若 Th = Tc ,移動 Q 時 W = 0 ( COP = ) 。

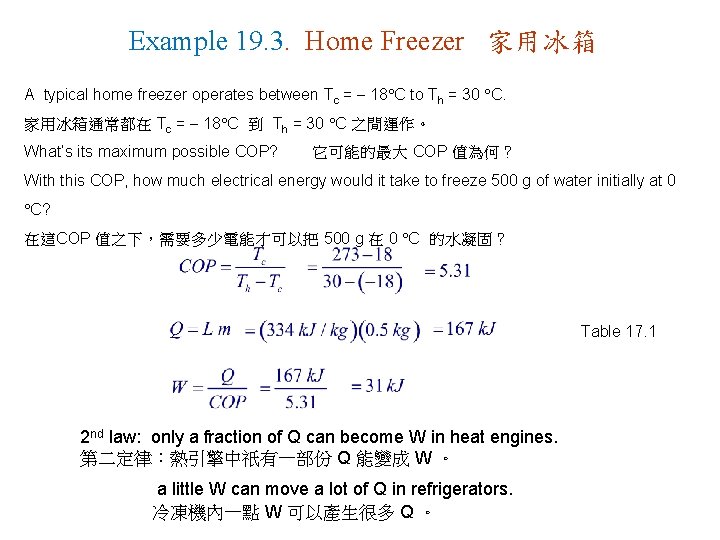
Example 19. 3. Home Freezer 家用冰箱 A typical home freezer operates between Tc = 18 C to Th = 30 C. 家用冰箱通常都在 Tc = 18 C 到 Th = 30 C 之間運作。 What’s its maximum possible COP? 它可能的最大 COP 值為何? With this COP, how much electrical energy would it take to freeze 500 g of water initially at 0 C? 在這COP 值之下,需要多少電能才可以把 500 g 在 0 C 的水凝固? Table 17. 1 2 nd law: only a fraction of Q can become W in heat engines. 第二定律:熱引擎中祇有一部份 Q 能變成 W 。 a little W can move a lot of Q in refrigerators. 冷凍機內一點 W 可以產生很多 Q 。

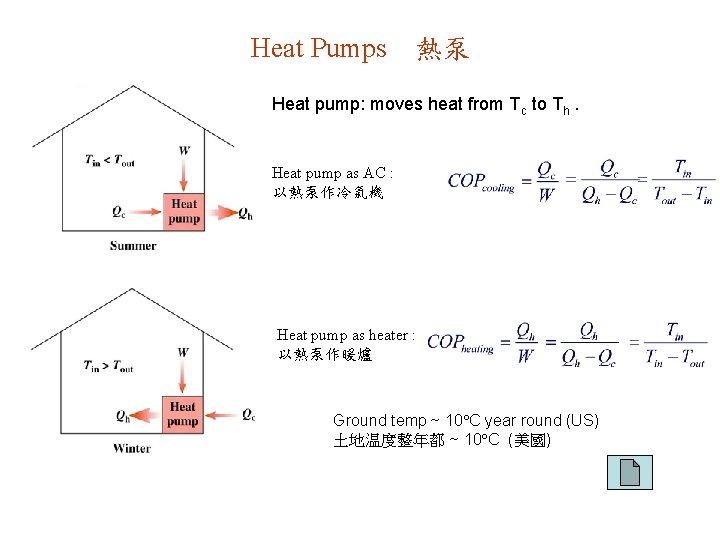
Heat Pumps 熱泵 Heat pump: moves heat from Tc to Th. Heat pump as AC : 以熱泵作冷氣機 Heat pump as heater : 以熱泵作暖爐 Ground temp ~ 10 C year round (US) 土地温度整年都 ~ 10 C (美國)

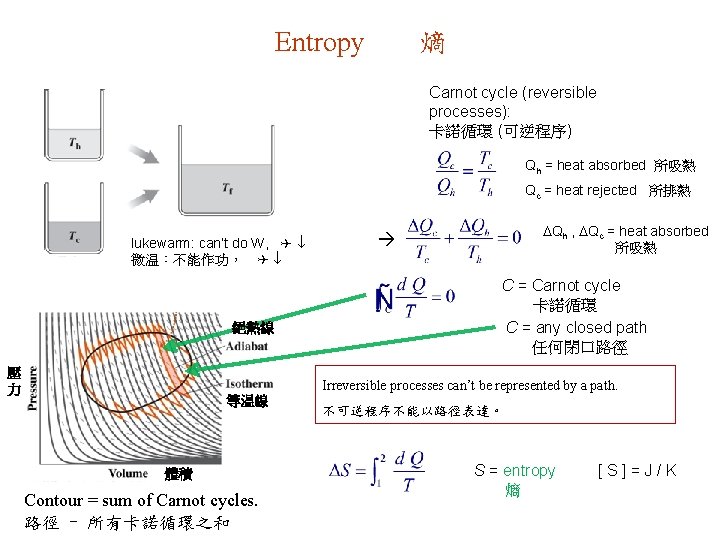
Entropy 熵 Carnot cycle (reversible processes): 卡諾循環 (可逆程序) Qh = heat absorbed 所吸熱 Qc = heat rejected 所排熱 lukewarm: can’t do W, Q 微温:不能作功, Q 絕熱線 壓 力 Qh , Qc = heat absorbed 所吸熱 C = Carnot cycle 卡諾循環 C = any closed path 任何閉口路徑 Irreversible processes can’t be represented by a path. 等温線 體積 Contour = sum of Carnot cycles. 路徑 = 所有卡諾循環之和 不可逆程序不能以路徑表達。 S = entropy 熵 [S]=J/K

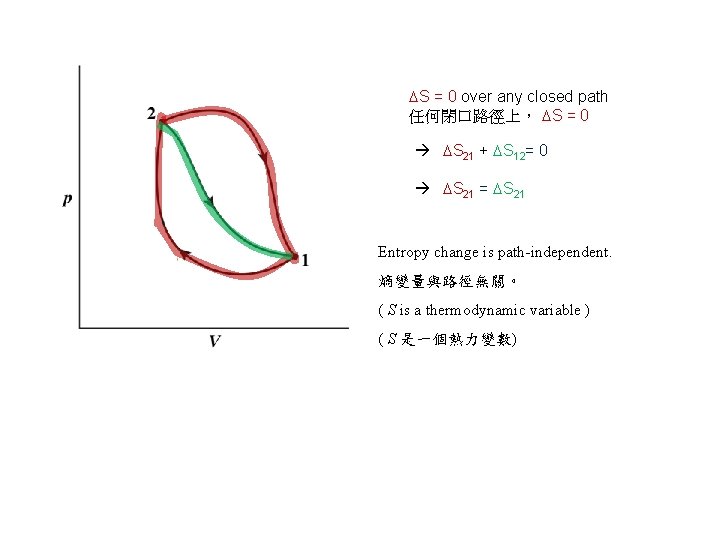
S = 0 over any closed path 任何閉口路徑上, S = 0 S 21 + S 12= 0 S 21 = S 21 Entropy change is path-independent. 熵變量與路徑無關。 ( S is a thermodynamic variable ) ( S 是一個熱力變數)

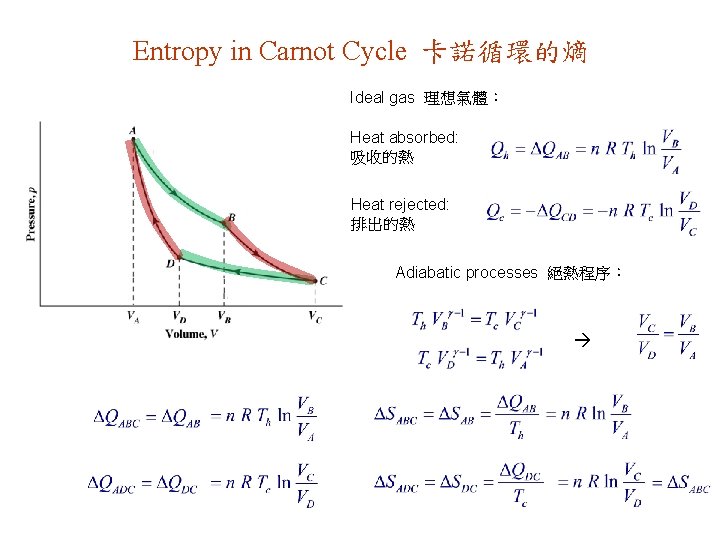
Entropy in Carnot Cycle 卡諾循環的熵 Ideal gas 理想氣體: Heat absorbed: 吸收的熱 Heat rejected: 排出的熱 Adiabatic processes 絕熱程序:

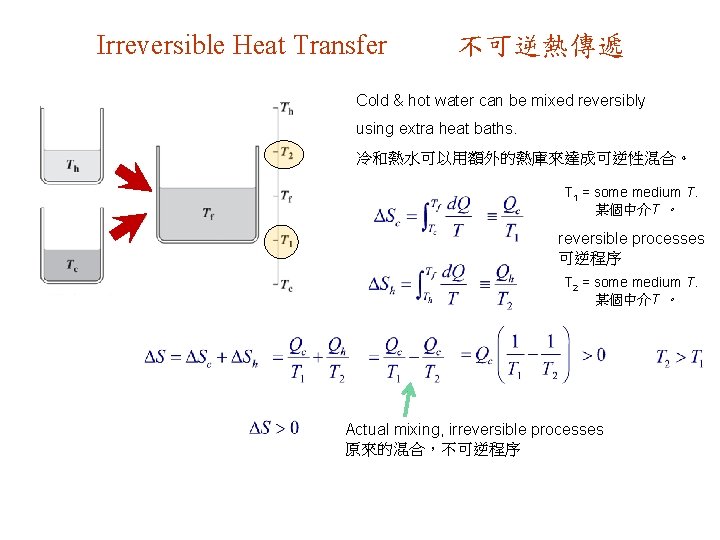
Irreversible Heat Transfer 不可逆熱傳遞 Cold & hot water can be mixed reversibly using extra heat baths. 冷和熱水可以用額外的熱庫來達成可逆性混合。 T 1 = some medium T. 某個中介T 。 reversible processes 可逆程序 T 2 = some medium T. 某個中介T 。 Actual mixing, irreversible processes 原來的混合,不可逆程序

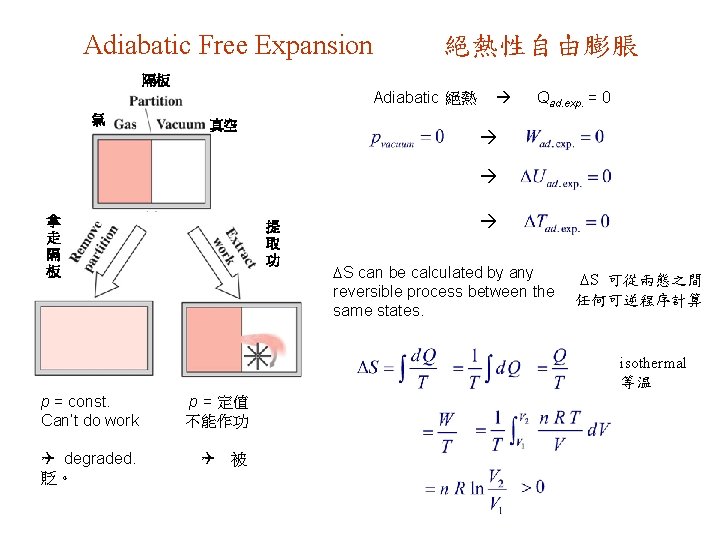
Adiabatic Free Expansion 絕熱性自由膨脹 隔板 Adiabatic 絕熱 氣 真空 Qad. exp. = 0 拿 走 隔 板 提 取 功 S can be calculated by any reversible process between the same states. S 可從兩態之間 任何可逆程序計算 isothermal 等温 p = const. Can’t do work Q degraded. 貶。 p = 定值 不能作功 Q 被

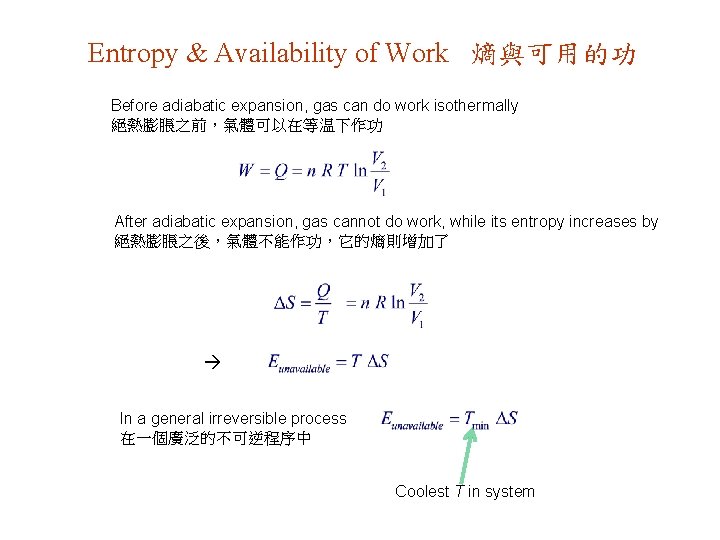
Entropy & Availability of Work 熵與可用的功 Before adiabatic expansion, gas can do work isothermally 絕熱膨脹之前,氣體可以在等温下作功 After adiabatic expansion, gas cannot do work, while its entropy increases by 絕熱膨脹之後,氣體不能作功,它的熵則增加了 In a general irreversible process 在一個廣泛的不可逆程序中 Coolest T in system

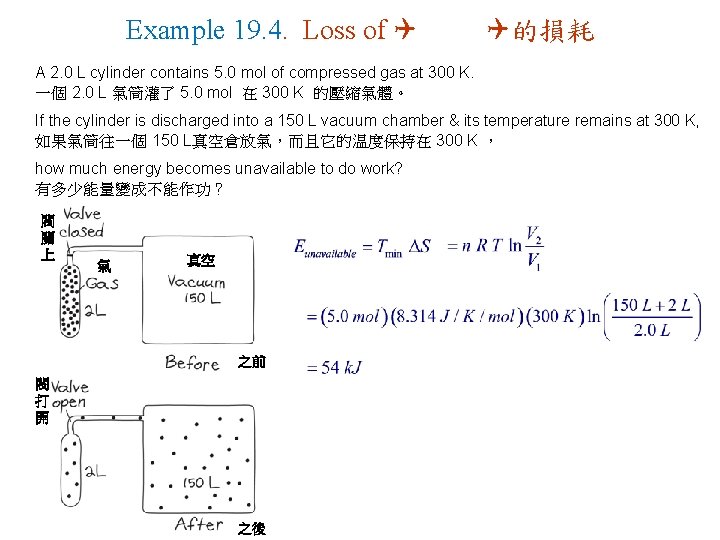
Example 19. 4. Loss of Q Q的損耗 A 2. 0 L cylinder contains 5. 0 mol of compressed gas at 300 K. 一個 2. 0 L 氣筒灌了 5. 0 mol 在 300 K 的壓縮氣體。 If the cylinder is discharged into a 150 L vacuum chamber & its temperature remains at 300 K, 如果氣筒往一個 150 L真空倉放氣,而且它的温度保持在 300 K , how much energy becomes unavailable to do work? 有多少能量變成不能作功? 閥 關 上 氣 真空 之前 閥 打 開 之後

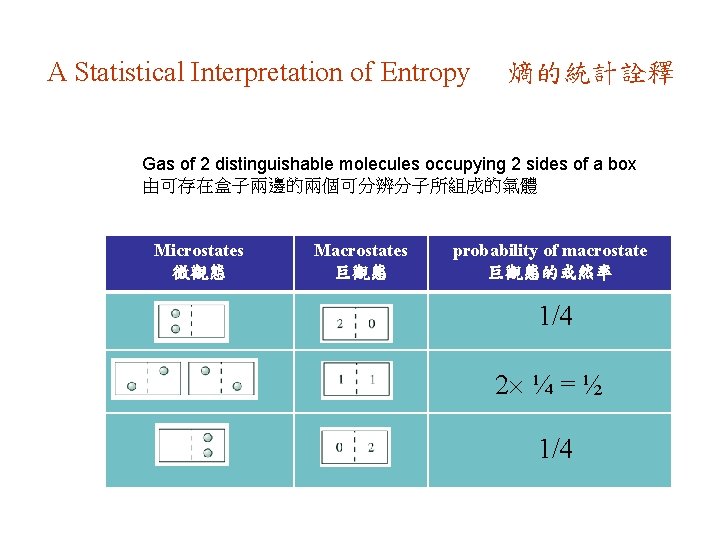
A Statistical Interpretation of Entropy 熵的統計詮釋 Gas of 2 distinguishable molecules occupying 2 sides of a box 由可存在盒子兩邊的兩個可分辨分子所組成的氣體 Microstates 微觀態 Macrostates 巨觀態 probability of macrostate 巨觀態的或然率 1/4 2 ¼ = ½ 1/4

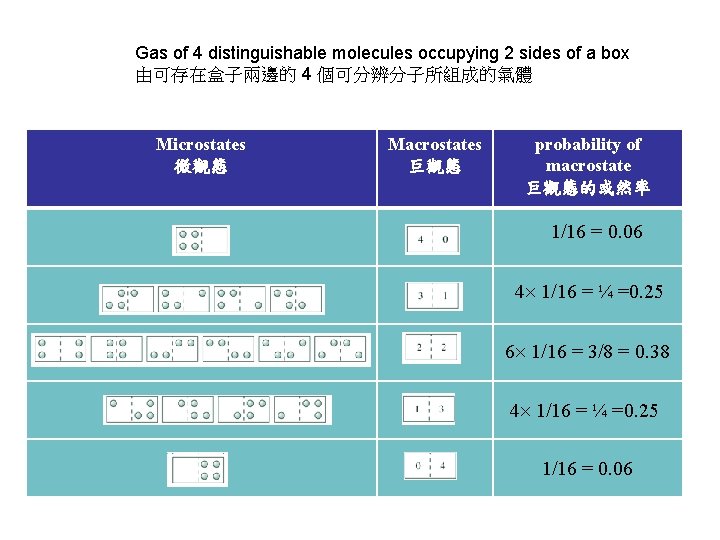
Gas of 4 distinguishable molecules occupying 2 sides of a box 由可存在盒子兩邊的 4 個可分辨分子所組成的氣體 Microstates 微觀態 Macrostates 巨觀態 probability of macrostate 巨觀態的或然率 1/16 = 0. 06 4 1/16 = ¼ =0. 25 6 1/16 = 3/8 = 0. 38 4 1/16 = ¼ =0. 25 1/16 = 0. 06

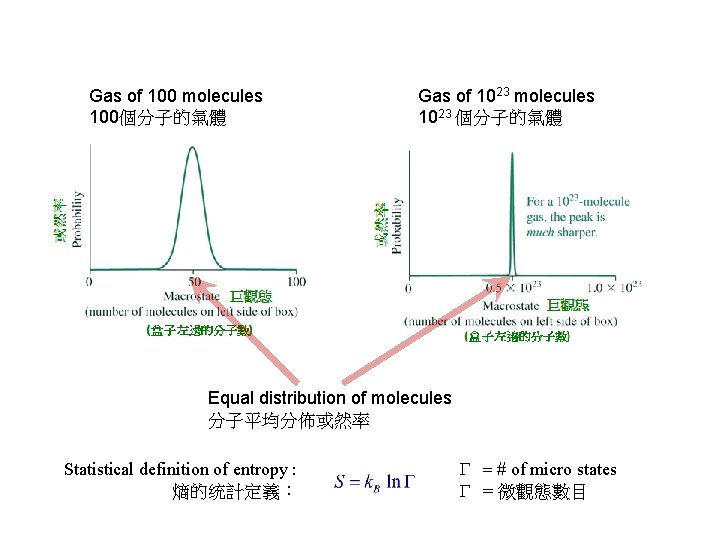
Gas of 100 molecules 100個分子的氣體 Gas of 1023 molecules 1023 個分子的氣體 Equal distribution of molecules 分子平均分佈或然率 Statistical definition of entropy : 熵的统計定義: G # of micro states G = 微觀態數目

Entropy & the 2 nd Law of Thermodynamics 熵與熱力學第二定律 2 nd Law of Thermodynamics : 熱力學第二定律 in any closed system 任何密閉系統中 S can decrease in an open system by outside work on it. S 可以在一個開放系統中因外界對它作功而減少。 However, S 0 for combined system. 不過,如果把”外界”也算進來,整個系统還是 S 0 。 S 0 in the universe 整個宇宙 S 0 Universe tends to disorder 宇宙趨向混亂 Life 生命 ?
 Long and short
Long and short Jack in the beanstalk climax
Jack in the beanstalk climax Usuf thermodynamics
Usuf thermodynamics Zeroth law example
Zeroth law example Newtons third law of thermodynamics
Newtons third law of thermodynamics Thermodynamics laws
Thermodynamics laws First law of thermodynamics
First law of thermodynamics First law of thermodynamics cyclic process
First law of thermodynamics cyclic process Isentropic compressor
Isentropic compressor Zeroth law of thermodynamics
Zeroth law of thermodynamics Joule's experiment in thermodynamics
Joule's experiment in thermodynamics Internal energy in thermodynamics definition
Internal energy in thermodynamics definition Steady flow process in thermodynamics
Steady flow process in thermodynamics Second law of thermodynamics
Second law of thermodynamics Thermodynamics rules
Thermodynamics rules Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Third law of thermodynamics is depend on
Third law of thermodynamics is depend on Dq=cdt
Dq=cdt First law of thermodynamics sign convention
First law of thermodynamics sign convention 1st law of thermodynamics
1st law of thermodynamics 1st law of thermodynamics
1st law of thermodynamics First law of thermodynamics control mass
First law of thermodynamics control mass Zeroth law of thermodynamics
Zeroth law of thermodynamics Free energy
Free energy 2 nd law of thermodynamics
2 nd law of thermodynamics