Sea Water Chemistry Chapter 7 Sea Water Chemistry











































- Slides: 52

Sea Water Chemistry Chapter 7

Sea Water Chemistry determine many important oceanographic phenomena including : n Global patterns of oceanic and atmospheric circulation, and the growth and distribution of marine organisms. n

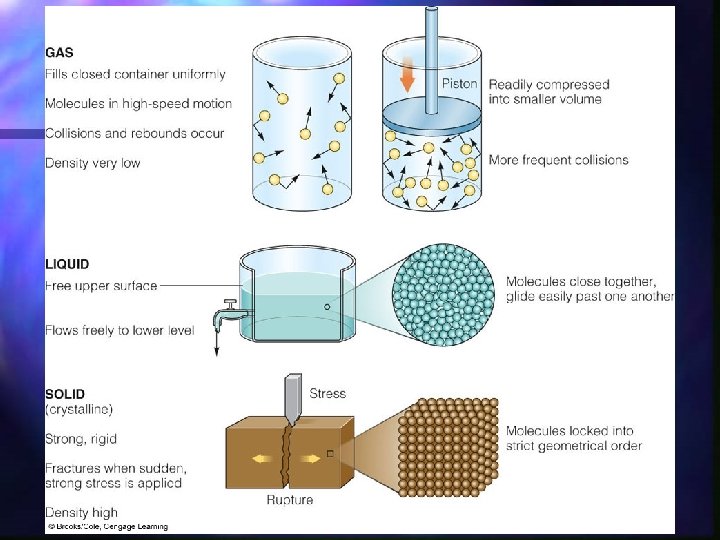
Life on earth probably evolved in water n Most animals are 50 -65% water n water exists in all three physical states of matter: solid, liquid, and gas n

71% of the Earth Surface - Sea Water n regulates the climate, dilute waste n major habitat for living creatures n

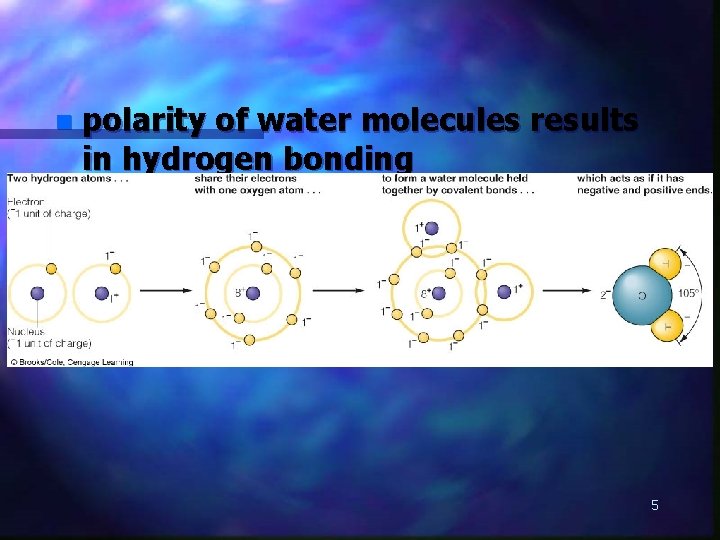
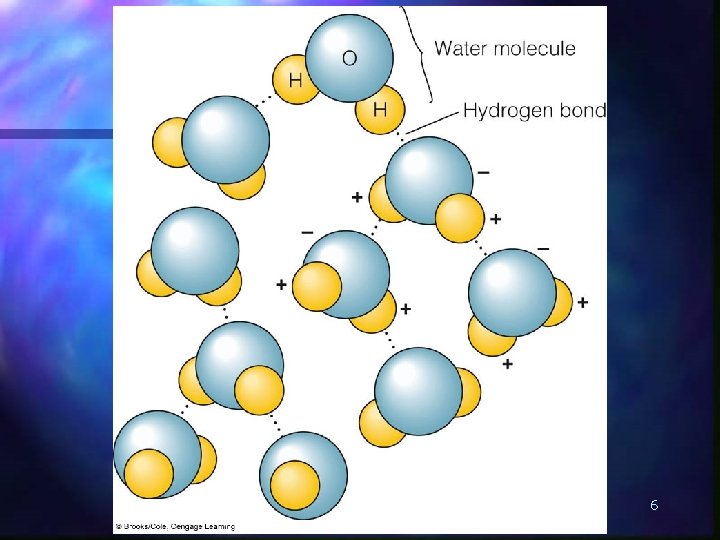
n polarity of water molecules results in hydrogen bonding 5

6

Characteristics of H 20 1. has cohesive behavior n 2. resists changes in temperature n 3. has a high heat of vaporization and cools surfaces as it evaporates n 4. expands when it freezes n 5. is a versatile solvent n 7


n 1. Surface tension – measure of how difficult it is to stretch or break the surface of a liquid – water has a greater surface tension than most liquids 9

2 -3. Water's high heat of vaporization: n moderates the earth's climate. n solar heat absorbed by tropical seas dissipates when surface water evaporates n 10


4. Oceans and lakes don't freeze n because of hydrogen bonding, water is less dense as a solid than it is as a liquid. n consequently, ice floats. n 11/3/2020 12

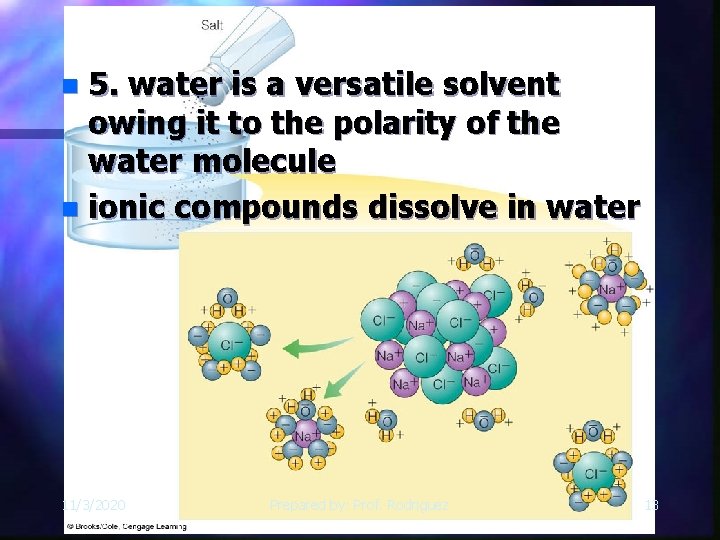
5. water is a versatile solvent owing it to the polarity of the water molecule n ionic compounds dissolve in water n 11/3/2020 Prepared by: Prof. Rodriguez 13

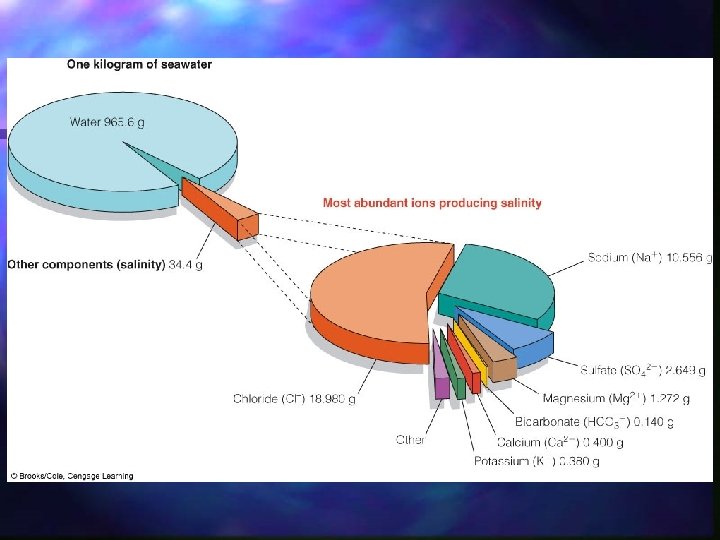
Major Components of Seawater dissolved salts - hydrated anions and cations (Table 7. 1; , f. 7. 3) n dissolved gases - nitrogen, oxygen, carbon dioxide n organic and inorganic - dissolved organic materials suspended particulate matter n

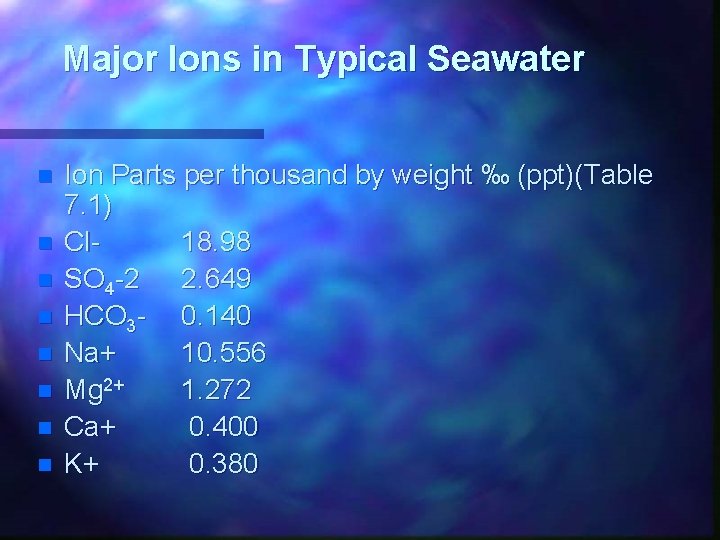
Major Ions in Typical Seawater n n n n Ion Parts per thousand by weight ‰ (ppt)(Table 7. 1) Cl 18. 98 SO 4 -2 2. 649 HCO 3 - 0. 140 Na+ 10. 556 Mg 2+ 1. 272 Ca+ 0. 400 K+ 0. 380



n n n On average, concentration of dissolved salts, i. e. , the salinity, in seawater is 3. 5% or 35‰. The relative abundances of the ions listed above does not change, even though salinity does; are said to be conservative. Relative abundances of minor and trace constituents do vary


Determining Salinity n n n Evaporation of water to weight the salt is an imprecise method Because of the constancy of composition if we measure one component we can get a more precise measurement Salinity ppt = 1. 80655 x Chlorinity in ppt If chlorinity is 19. 2 ppt, what is the salinity of sea water? 34. 7 ppt = 35 ppt

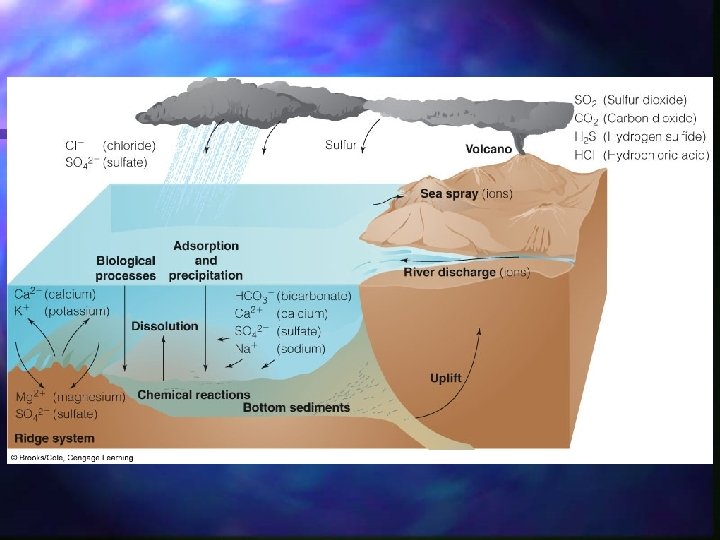
Sources of Salt Rivers (winds and glaciers are a less important, indirect source) n Weathering of oceanic crust n Hydrothermal Vents associated with Mid -ocean ridges and other submarine volcanoes n

Sinks Biologic activity n Interaction with Particulate matter: clays and organic matter absorb dissolved metals n

Direct Precipitation Hydrothermal Activity: (fig. 7. 4) n Reaction between seawater and new oceanic crust n Minerals like magnesium is incorporated into deposits n Calcium is added to sea water n

Physical and Chemical Properties of Water Heat Capacity - energy added to raise temperature of 1 gram of substance by 1°C n adding energy breaks H-bonds, increases fraction of free water n important in thermal buffering and heat transport to higher latitudes n

Latent Heats and Evaporation heat input or release associated with phase changes (ice - liquid, liquid vapor) n changes in water structure, H-bonding with phase changes n important in thermal buffering, heat transport and heat exchange with atmosphere n

Density - mass per unit volume (grams/cm 3) n density of water phases (ice, liquid, vapor) due to structural changes at molecular level n density maximum at 4°C in pure water n Major role in deep ocean circulation and water column structure and stability n


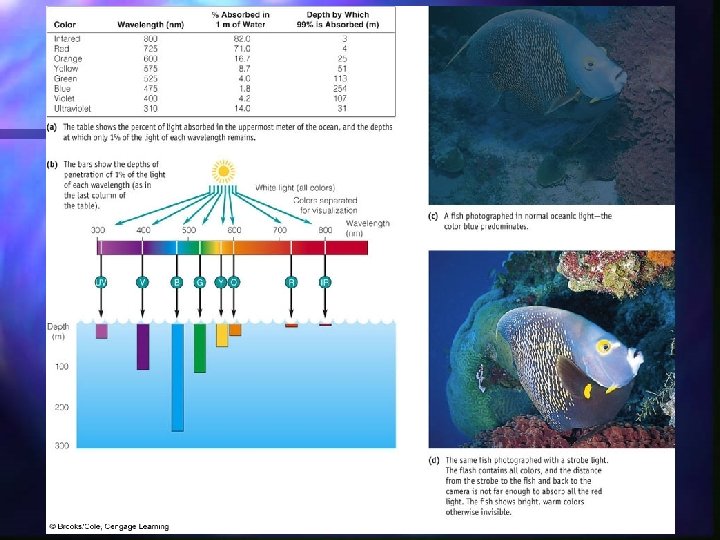
Light Transmission transparent in visible part of spectrum n Absorbed as is goes deeper in the water column n strongly absorbs infrared (heat) and ultraviolet (prevents damage to DNA) n


Dissolving Power hydration of solutes - interactions between solutes and free water n decreases H-bonding, increases order of free water, increases density n exclusion of solutes on freezing and evaporation n other effects of solutes: freezing point depression, boiling point elevation n

p. H (acidity or alkalinity) n measure of the dissociation of water into ions (H+, OH), (fig. 7. 9) n p. H n = - log [H+] p. H effects on biological and geochemical reactions



Conservative vs. Non Conservative Properties of Seawater n those properties that can only be altered at the sea surface: temperature, salinity, inert gases n properties not altered by biological or geochemical reactions n importance in water mass identification, tracing and mixing n

Nonconservative Properties of Seawater n those properties that can be altered anywhere in the water column n properties altered by biological and geochemical reactions n

Dissolved Gases n n n The proportions of gases in the atmosphere is not the same as their proportions in seawater, (Table 7. 4) There is less N 2 (nitrogen gas) in the ocean than in the atmosphere, much more oxygen, and even more CO 2. All this CO 2 in the oceans keeps CO 2 from being in the atmosphere and causing global warming.


The colder the water, the more gas can dissolve in it. n When you leave your can of coke in the car in the sun, then open it, what happens? n Coke sprays all over you. n That's because the gas has exsolved (come out of solution); a lot has accumulated in the little space at the top of the can. n

n n Very active fish, such as trout and salmon, require very cold water to live in because they have high oxygen requirements. They literally suffocate when the water gets too warm, and the oxygen levels drop. This explain why these fish don't live down south. 'Thermal pollution' occurs when electric plants put warm water into streams, lowering the oxygen level.

CO 2 is important because it is needed by plants so they can photosynthesize. n O 2 is important because animals need it for respiration. n Photosynthesis: n CO 2 + H 2 O + energy [from the sun] O 2 + sugar (organic matter) Respiration (the reverse of photosynthesis): n O 2 + sugar CO 2 + H 2 O + energy

Dissolved Oxygen seawateratmosphere exchange at air water interface only (fig. 7. 8) n biological processes that affect O 2 concentration: photosynthesis and respiration n typical distribution of O 2 in water column and processes that control this distribution n

Phytoplankton Nutrients inorganic sources of N, P, S and other atoms required for phytoplankton growth n photosynthesis and respiration contributes in nutrient distribution n

n n Especially important, because so much is needed, are N (nitrogen) and P (phosphorus). Si (silica) is also important for all the siliceous organisms we‘ll discuss: diatoms, radiolarians, and siliceous sponges. N is necessary to make proteins. P is necessary to make new cells (it's part of the cell wall), and also genetic material, DNA and RNA.

n n n n N is useful for plants only in these forms: NO 3 - nitrate NO 2 - nitrite NH 4+ ammonium N 2, the gas, is not usable by most plants. Only a few bacteria can break this very strong molecule apart and turn it into nitrate. These are 'nitrogen-fixing bacteria'. P is useful in the form of phosphate, PO 43 -

Thus weathering, sedimentation, and ocean chemistry are all closely linked. n Other ions in seawater, such as Cl – and SO 4 , are not derived from weathering, but from volcanic degassing. n

The Carbonate System in Seawater CO 2 in seawater is controlled by: ( f. 7 -10 -11) n Exchange with the atmosphere n Photosynthesis/Respiration: n 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + 6 O 2 n

The Carbonate Buffer Carbon Dioxide: CO 2 , n Carbonic Acid: H 2 CO 3 , n Bicarbonate: HCO 3 - , n Carbonate: CO 32 n CO 2 + H 2 O H 2 CO 3. n H 2 CO 3 HCO 3 - + H+2. n HCO 3 CO 32 - + H +3. n


n n n Another important reaction is the dissolution and precipitation of calcium carbonate: CO 3 + Ca+2 Ca. CO 34. . Importance of these reactions: Maintain constant p. H (seawater is said to be buffered). Few marine organisms can tolerate a p. H very different from 8.

Biological Productivity In general, shells of organisms are likely to be preserved where their production rate is high, n Siliceous shells are preserved only where the production rate is high. n Siliceous oozes occur where productivity rate is high and terrigenous sedimentation rate low. n

n n Carbonate shells: the oceans are supersaturated with respect to Ca. CO 3 at the surface and become increasingly undersaturated with depth. Shells more likely to be preserved at shallow depth. Lysocline: depth at which rapid dissolution of Ca. CO 3 begins. This is deeper than the depth where ocean becomes undersaturated.

CCD Carbonate Compensation Depth (or ‘snow line’): n depth where dissolution>supply of Ca. CO 3 and n below which Ca. CO 3 shells are not preserved in sediment. n
 Water and water and water water
Water and water and water water Led soldiers across hellespont into anatolia goals
Led soldiers across hellespont into anatolia goals Yellow sea and east china sea
Yellow sea and east china sea Anemones phylum
Anemones phylum What does the sea symbolize in the old man and the sea
What does the sea symbolize in the old man and the sea Sea stack diagram
Sea stack diagram Class 8 english chapter 7 water water everywhere
Class 8 english chapter 7 water water everywhere Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Physical properties of seawater
Physical properties of seawater Alex tickles his brother by stroking adjacent
Alex tickles his brother by stroking adjacent Density of sea water
Density of sea water Importance of forecasting
Importance of forecasting Rutgers sea surface temps hudson canyon
Rutgers sea surface temps hudson canyon Bromine in seawater
Bromine in seawater Sea water air conditioning
Sea water air conditioning Stone canal starfish
Stone canal starfish Chapter 9 surface water chapter assessment answer key
Chapter 9 surface water chapter assessment answer key Ocean water chemistry
Ocean water chemistry Chapter 6 chemistry in biology
Chapter 6 chemistry in biology The village by the sea chapter 1
The village by the sea chapter 1 Sea of monsters chapter 1 summary
Sea of monsters chapter 1 summary What impact did nearness to the sea have on greece
What impact did nearness to the sea have on greece Drinking fountain
Drinking fountain Fwa kapal
Fwa kapal Water o water
Water o water Estimating products of fractions
Estimating products of fractions Water exchanger
Water exchanger Fresh water meets salt water
Fresh water meets salt water Warm water rises in a lake. cold water descends.
Warm water rises in a lake. cold water descends. Clil water
Clil water Importance of water management
Importance of water management Unit 11 water water everywhere
Unit 11 water water everywhere Is chalk natural or manmade
Is chalk natural or manmade Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Introduction to organic chemistry
Introduction to organic chemistry Chapter 9 review stoichiometry section 1 answer key
Chapter 9 review stoichiometry section 1 answer key Chemical formula of love
Chemical formula of love Modern chemistry chapter 15
Modern chemistry chapter 15 Chapter 14 review acids and bases section 1
Chapter 14 review acids and bases section 1 Modern chemistry chapter 13
Modern chemistry chapter 13 Chapter 12 solutions chemistry
Chapter 12 solutions chemistry Chemistry chapter 11 gases test
Chemistry chapter 11 gases test Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 chemistry study guide
Chapter 10 chemistry study guide Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas Chapter 7 organic chemistry
Chapter 7 organic chemistry Chapter 24: nuclear chemistry answer key
Chapter 24: nuclear chemistry answer key Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers All acids owe their chemical reactivity to
All acids owe their chemical reactivity to