Water Hardness Water Hardness Hard water is water







- Slides: 14

Water Hardness

Water Hardness � Hard water is water that will not easily form a lather with soap. � Hardness is caused by the presence of Ca 2+ or Mg 2+ ions dissolved in the water. These ions react with the soap to form a scum rather than a lather. � The concentration of the Ca 2+ ions is greater than the concentration of any other metal ion in our water. � Water hardness is usually expressed in ppm Ca. CO 3

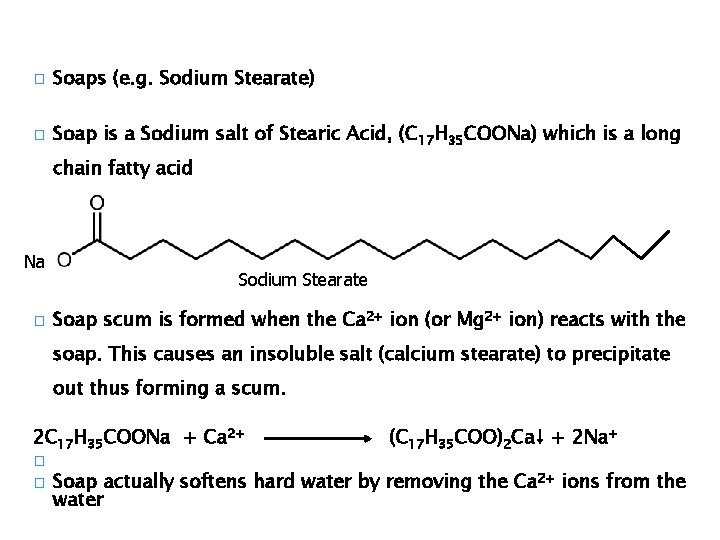
� Soaps (e. g. Sodium Stearate) � Soap is a Sodium salt of Stearic Acid, (C 17 H 35 COONa) which is a long chain fatty acid Na � Sodium Stearate Soap scum is formed when the Ca 2+ ion (or Mg 2+ ion) reacts with the soap. This causes an insoluble salt (calcium stearate) to precipitate out thus forming a scum. 2 C 17 H 35 COONa + Ca 2+ � � (C 17 H 35 COO)2 Ca↓ + 2 Na+ Soap actually softens hard water by removing the Ca 2+ ions from the water

Types of Hardness � Temporary Hardness � Permanent Hardness

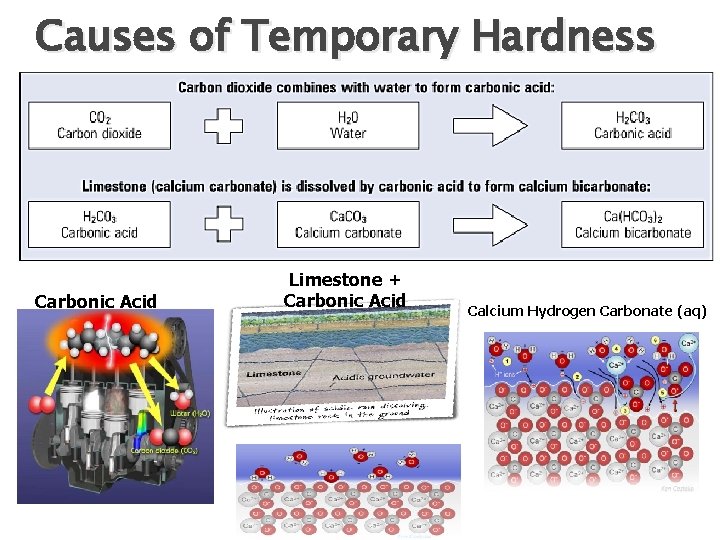
Temporary Hardness is hardness that can be removed by boiling. Temporary hardness is caused by: ◦ Calcium Hydrogencarbonate Ca(HCO 3)2 ◦ Magnesium Hydrogencarbonate Mg(HCO 3)2

Water Cycle

Causes of Temporary Hardness Carbonic Acid Limestone + Carbonic Acid Calcium Hydrogen Carbonate (aq)

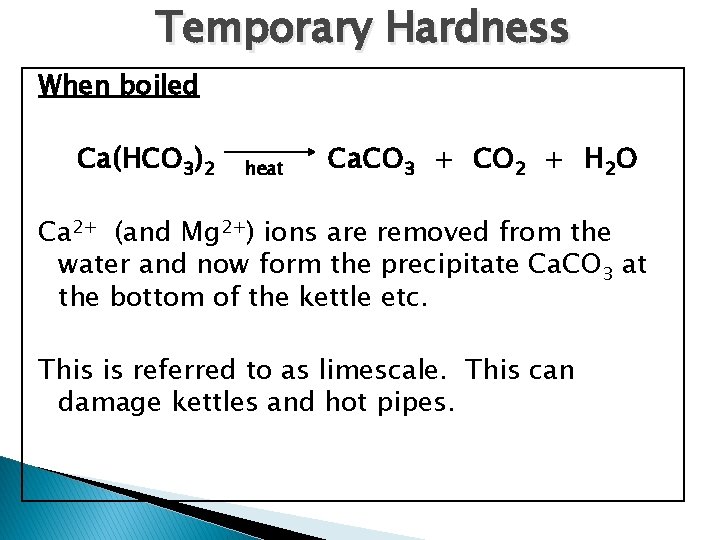
Temporary Hardness When boiled Ca(HCO 3)2 heat Ca. CO 3 + CO 2 + H 2 O Ca 2+ (and Mg 2+) ions are removed from the water and now form the precipitate Ca. CO 3 at the bottom of the kettle etc. This is referred to as limescale. This can damage kettles and hot pipes.

Why Be Concerned About Hard Water? � Hard water does cause soap scum, clogs pipes and clogs boilers as limescale

Permanent hardness � Permanent Hardness is hardness that cannot be removed by boiling. � Permanent hardness is caused by ◦ Calcium Chloride Ca. Cl 2 ◦ Magnesium Chloride Mg. Cl 2 ◦ Calcium Sulfate Ca. SO 4 ◦ Magnesium Chloride Mg SO 4

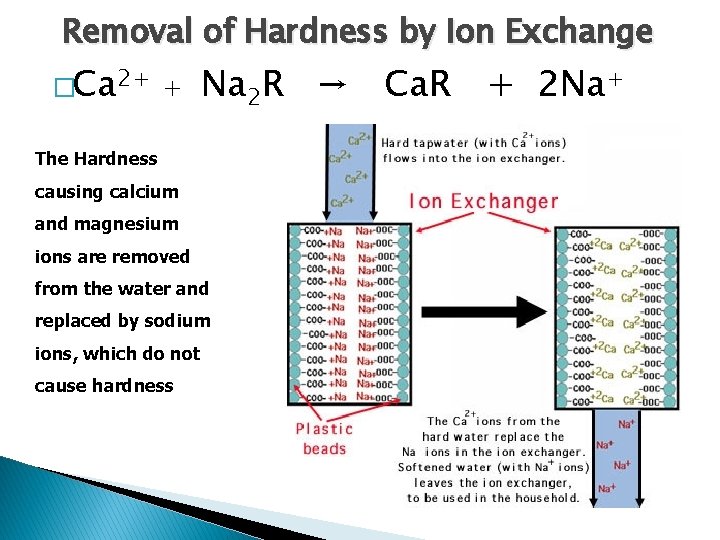
Removal of Hardness by Ion Exchange �Ca 2+ + Na 2 R → Ca. R + 2 Na+ The Hardness causing calcium and magnesium ions are removed from the water and replaced by sodium ions, which do not cause hardness

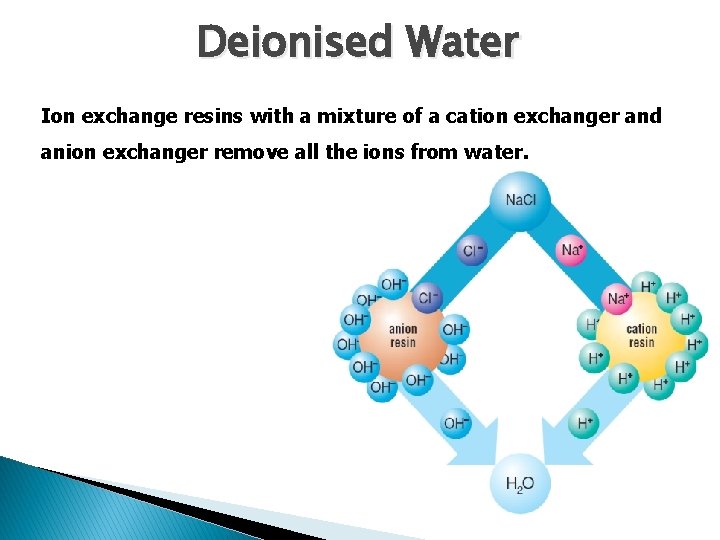
Deionised Water Ion exchange resins with a mixture of a cation exchanger and anion exchanger remove all the ions from water.

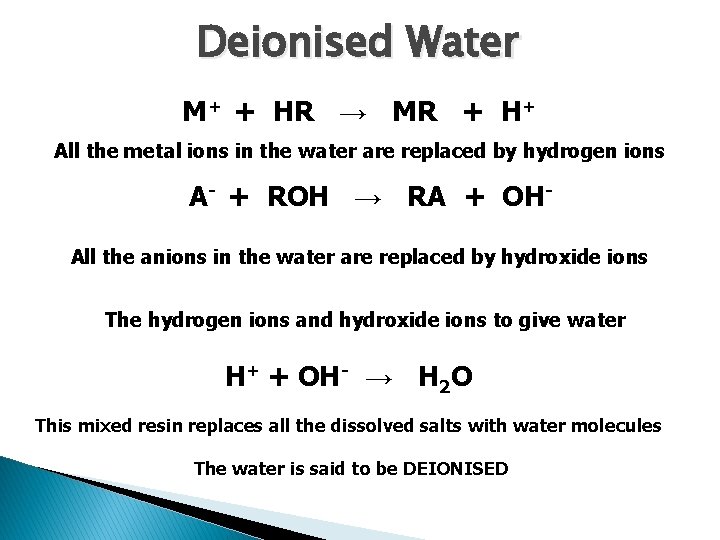
Deionised Water M+ + HR → MR + H+ All the metal ions in the water are replaced by hydrogen ions A- + ROH → RA + OHAll the anions in the water are replaced by hydroxide ions The hydrogen ions and hydroxide ions to give water H+ + OH- → H 2 O This mixed resin replaces all the dissolved salts with water molecules The water is said to be DEIONISED

Deionised Water and Distilled Water Deionised water is not quite as pure as distilled water Deionised water has all the dissolved ions in the water removed Distilled water has all the dissolved solids and dissolved gases removed
 T.me/makeshard
T.me/makeshard Pseudo hardness of water
Pseudo hardness of water Zéolite danger
Zéolite danger What does hard water mean
What does hard water mean Units of hardness of water
Units of hardness of water How to calculate hardness of water
How to calculate hardness of water What information should a master cleaning schedule contain?
What information should a master cleaning schedule contain? Causes of hardness
Causes of hardness Removal of water hardness
Removal of water hardness Knockhardy gcse
Knockhardy gcse Work hard. have fun. make history
Work hard. have fun. make history Hard times hard drive
Hard times hard drive Water and water and water water
Water and water and water water Permanent hardness is also called as
Permanent hardness is also called as Helium physical and chemical properties
Helium physical and chemical properties