Water hardness Effect of water hardness lime deposition







- Slides: 12

Water hardness: • Effect of water hardness: lime deposition (washing machine, boilers) - soap is less effective

Determination of water hardness: Total hardness: the amount of calcium and magnesium salts. These elements naturally enter the water through the dissolving action of carbonic acid or biochemical processes in the soil. The hardness of natural origin can change under the influence of different types of wastewater. – The temporary hardness (carbonate hardness) is the amount of calcium bicarbonate (Ca (HCO 3) 2) and a magnesium bicarbonate (Mg (HCO 3) 2) – The permanent hardness (noncarbonate hardness) is the difference between total and carbonate hardness. It is given by the amount of other calcium and magnesium salts.

temporary hardness: At high temperatures, the balance shifts to the right, therefore The variable hardness can be removed by boiling.

Since calcium carbonate is one of the more common causes of hardness, total hardness is usually reported in terms of calcium carbonate concentration (mg/L as Ca. CO 3),

Determination of water hardness: A groundwater has the following analysis: • calcium 75 mg/L • Magnesium : 40 mg/L • Sodium: 10 mg/L • Bicarbonate: 300 mg/L • Chloride: 10 mg/L • Sulphate: 109 mg/L. Compute total hardness carbonate hardness and non carbonate hardness all expressed in mg/L Ca. CO 3 https: //keisan. casio. com/exec/system/1350974216


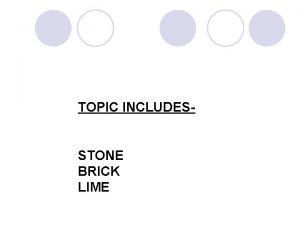
Homework 1.

Determination of total water hardness: eriochrome black T indicator, p. H =10 t

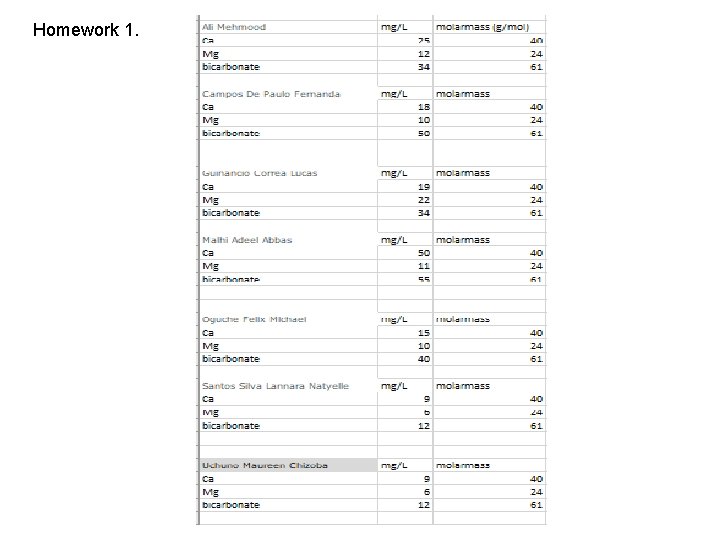
Ethylenediamine-teraacetic acid

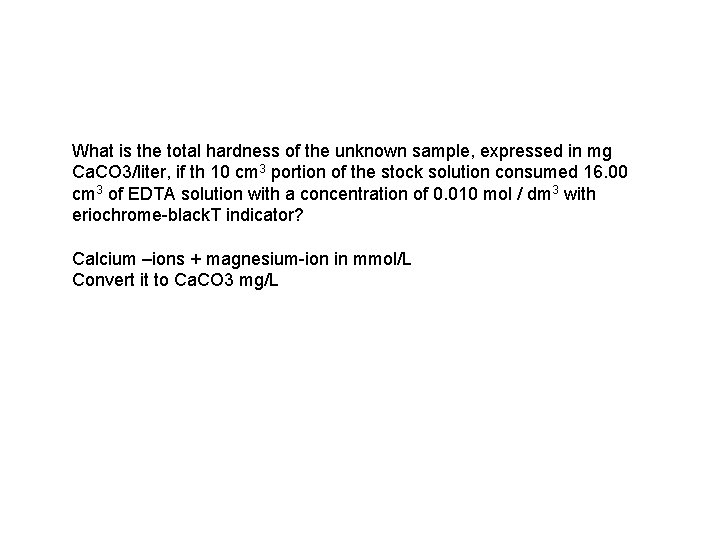
What is the total hardness of the unknown sample, expressed in mg Ca. CO 3/liter, if th 10 cm 3 portion of the stock solution consumed 16. 00 cm 3 of EDTA solution with a concentration of 0. 010 mol / dm 3 with eriochrome-black. T indicator? Calcium –ions + magnesium-ion in mmol/L Convert it to Ca. CO 3 mg/L

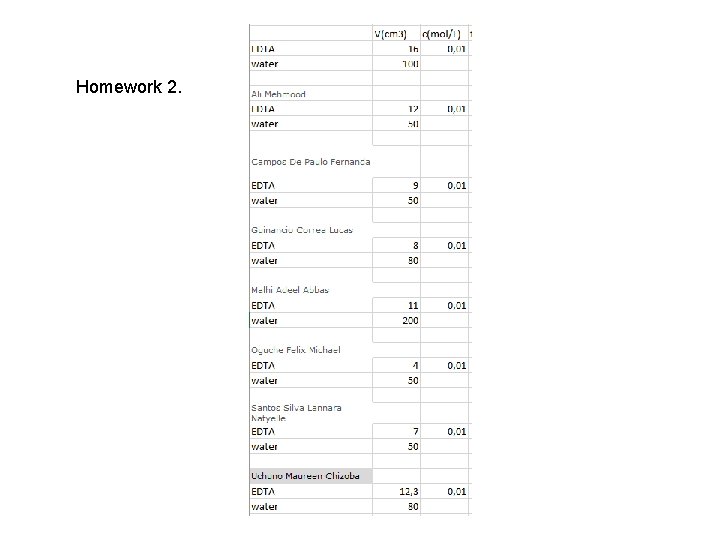
Homework 2.

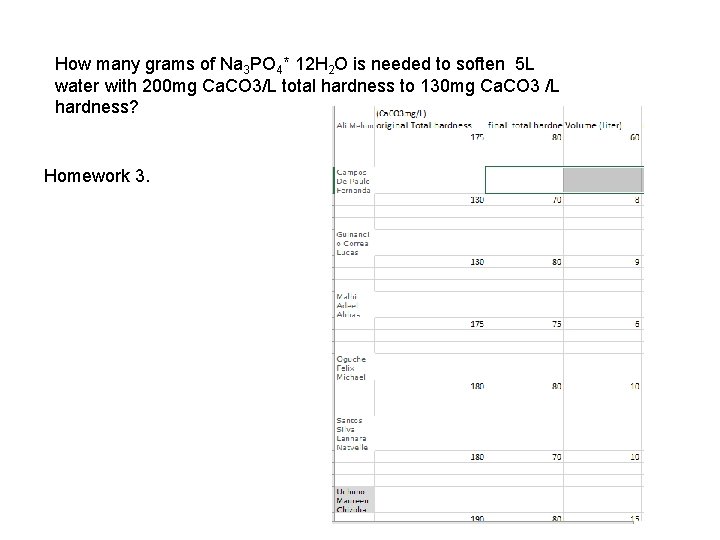
How many grams of Na 3 PO 4* 12 H 2 O is needed to soften 5 L water)with 200 mg Ca. CO 3/L total hardness to 130 mg Ca. CO 3 /L hardness? Homework 3.
 Differentiate between fat lime and hydraulic lime
Differentiate between fat lime and hydraulic lime Caustic embrittlement
Caustic embrittlement Lime soda process of water softening
Lime soda process of water softening Lime water milky
Lime water milky Lime water formula
Lime water formula Co2 and lime water equation
Co2 and lime water equation Warm sugar solution + yeast and lime water
Warm sugar solution + yeast and lime water Carbon dioxide gas is passed through lime water
Carbon dioxide gas is passed through lime water Water and water and water water
Water and water and water water How to calculate total hardness
How to calculate total hardness Water hardness equation
Water hardness equation Knockhardy gcse
Knockhardy gcse What does hard water mean
What does hard water mean