Hardness in Water Hard water is any water





![Temporary Hardness • Caused by dissolved calcium [or magnesium] hydrogencarbonate • When the water Temporary Hardness • Caused by dissolved calcium [or magnesium] hydrogencarbonate • When the water](https://slidetodoc.com/presentation_image_h/0d0bc22b9b7c75cbdefb89a5eedb3cd8/image-6.jpg)





![(3) Washing Soda [Calgon] Na 2 CO 3 • Sodium carbonate [Na 2 CO (3) Washing Soda [Calgon] Na 2 CO 3 • Sodium carbonate [Na 2 CO](https://slidetodoc.com/presentation_image_h/0d0bc22b9b7c75cbdefb89a5eedb3cd8/image-13.jpg)




![2. Flocculation • Al 2 SO 4 [ a Flocculating Agent] is added to 2. Flocculation • Al 2 SO 4 [ a Flocculating Agent] is added to](https://slidetodoc.com/presentation_image_h/0d0bc22b9b7c75cbdefb89a5eedb3cd8/image-19.jpg)






![Eutrophication Excess plant growth [algae] caused by excess nutrients in water. • Mainly caused Eutrophication Excess plant growth [algae] caused by excess nutrients in water. • Mainly caused](https://slidetodoc.com/presentation_image_h/0d0bc22b9b7c75cbdefb89a5eedb3cd8/image-28.jpg)






![E U Limits For levels of nitrate, phosphate and specific metal ions [2 examples] E U Limits For levels of nitrate, phosphate and specific metal ions [2 examples]](https://slidetodoc.com/presentation_image_h/0d0bc22b9b7c75cbdefb89a5eedb3cd8/image-35.jpg)












- Slides: 54

Hardness in Water

• Hard water is any water that forms a scum with soap. • Hard water does not form a lather with soap. • Soft water forms a lather with soap.

How is the Scum formed? • Soap is sodium stearate • All sodium salts are soluble in water so soap is soluble in water • Calcium or magnesium ions in the water react with soap ions [stearate ions] to form the insoluble scum [calcium stearate or magnesium stearate]

How do calcium ions get into the water?

• Carbon dioxide in the air reacts with water to form carbonic acid. • CO 2 + H 2 O = H 2 CO 3 • This is a weak acid and it reacts with limestone or marble [both are forms of calcium carbonate] • to form calcium hydrogencarbonate which is soluble and dissolves in the water. • Ca. CO 3 + H 2 CO 3 = Ca(HCO 3)2
![Temporary Hardness Caused by dissolved calcium or magnesium hydrogencarbonate When the water Temporary Hardness • Caused by dissolved calcium [or magnesium] hydrogencarbonate • When the water](https://slidetodoc.com/presentation_image_h/0d0bc22b9b7c75cbdefb89a5eedb3cd8/image-6.jpg)
Temporary Hardness • Caused by dissolved calcium [or magnesium] hydrogencarbonate • When the water is heated this breaks down to form calcium carbonate called limescale of furring. Ca(HCO 3)2(aq) = Ca. CO 3(s) + CO 2(g)+ H 2 O(l) To show that limescale is Ca. CO 3 • Add HCl and CO 2 produced – turns limewater milky

Problems caused by Temporary Hardness • Scum in laundries – specks on dark wool • Limescale or ‘furring’ in kettles & boilers. • Limescale is a bad conductor of heat so it also makes the boilers inefficient • Blocking pipes in boilers

Permanent Hardness • Hardness NOT removed by boiling • Caused by any or all of the following – – Calcium sulphate (Ca. SO 4) Magnesium sulphate (Mg. SO 4) Calcium chloride (Ca. Cl 2) Magnesium chloride (Mg. Cl 2) • Dissolved in the water from soil • It does not cause limescale but it does cause scum.

Removal of Hardness

(1) Boiling only removes temporary hardness Ca(HCO 3)2(aq) = Ca. CO 3(s) + CO 2(g) + H 2 O(l) (2) Ion exchange removes both Temporary and Permanent • Ca 2+ and Mg 2+ are exchanged for H+ • Cl-, SO 42 -, HCO 3 - are exchanged for OH • then H+ and OH- combine to form H 2 O. • Result is deionised water

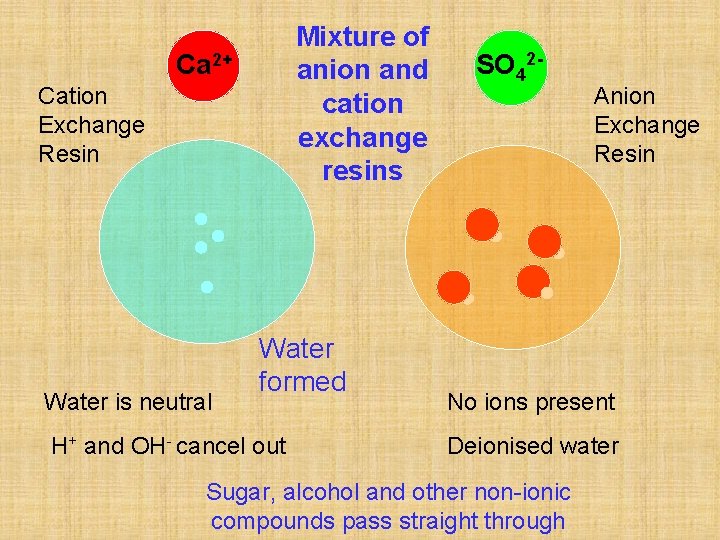
Mixture of anion and cation exchange resins Ca 2+ Cation Exchange Resin Water is neutral Water formed H+ and OH- cancel out SO 42 - Anion Exchange Resin No ions present Deionised water Sugar, alcohol and other non-ionic compounds pass straight through

Deionised v. Distilled Water • Deionised has • No ions • Can have dirt, bacteria, dissolved gases and • covalent solutes e. g. alcohol and sugar • Distilled water is completely pure • It is more expensive to prepare and it is often not worth the bother
![3 Washing Soda Calgon Na 2 CO 3 Sodium carbonate Na 2 CO (3) Washing Soda [Calgon] Na 2 CO 3 • Sodium carbonate [Na 2 CO](https://slidetodoc.com/presentation_image_h/0d0bc22b9b7c75cbdefb89a5eedb3cd8/image-13.jpg)
(3) Washing Soda [Calgon] Na 2 CO 3 • Sodium carbonate [Na 2 CO 3] is soluble like all sodium salts • Dissolve it in hard water [Temporary or Permanent] • CO 32 - Reacts with Ca 2+ [and Mg 2+] ions to form insoluble Ca. CO 3 as a very fine powder which cannot be seen • Ca 2+(aq) + CO 32 -(aq) = Ca. CO 3(s) • Mg 2+(aq) + CO 32 -(aq) = Mg. CO 3(s)

Water Treatment Making water safe to drink

• Water is one of the most dangerous substances on earth • Carries diseases e. g. • Cholera , typhus, schistasomiasis, polio, malaria [ mosquitoes breed in it], dysentery and lots of other parasites • Disease far more prevalent in the Third World • Because of unclean drinking water • Here water is free of disease and pollution • We treat our water very carefully before allowing it to be consumed.

Steps involved in purifying our water

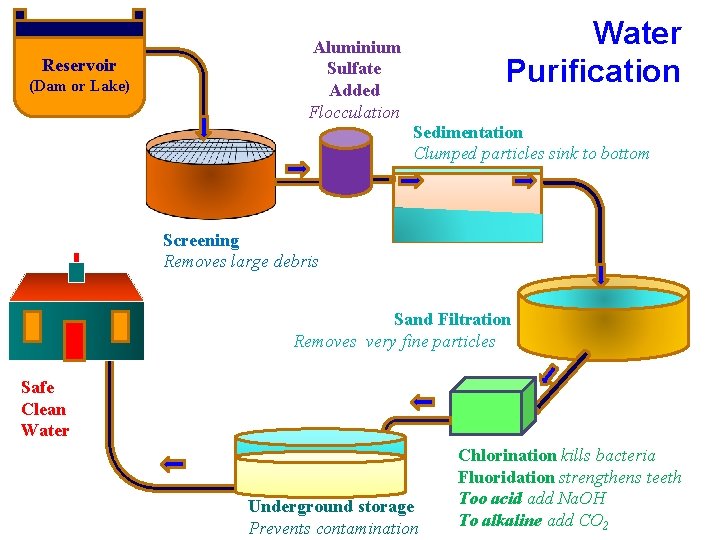
Reservoir (Dam or Lake) Water Purification Aluminium Sulfate Added Flocculation Sedimentation Clumped particles sink to bottom Screening Removes large debris Sand Filtration Removes very fine particles Safe Clean Water Underground storage Prevents contamination Chlorination kills bacteria Fluoridation strengthens teeth Too acid add Na. OH To alkaline add CO 2

1. Screening • Water is collected in reservoirs • People can dispose of all sorts of rubbish in the reservoirs either accidentally or on purpose • A large mesh is used to filter out dead sheep, goats and elephants • It also filters out babies nappies, coke bottles, sticks etc.
![2 Flocculation Al 2 SO 4 a Flocculating Agent is added to 2. Flocculation • Al 2 SO 4 [ a Flocculating Agent] is added to](https://slidetodoc.com/presentation_image_h/0d0bc22b9b7c75cbdefb89a5eedb3cd8/image-19.jpg)
2. Flocculation • Al 2 SO 4 [ a Flocculating Agent] is added to the water. • There are tiny particles of dirt suspended in the water which make it cloudy. • They are too small to sink to the bottom • The Al 2 SO 4 makes them clump together and sink to the bottom [Flocculation]

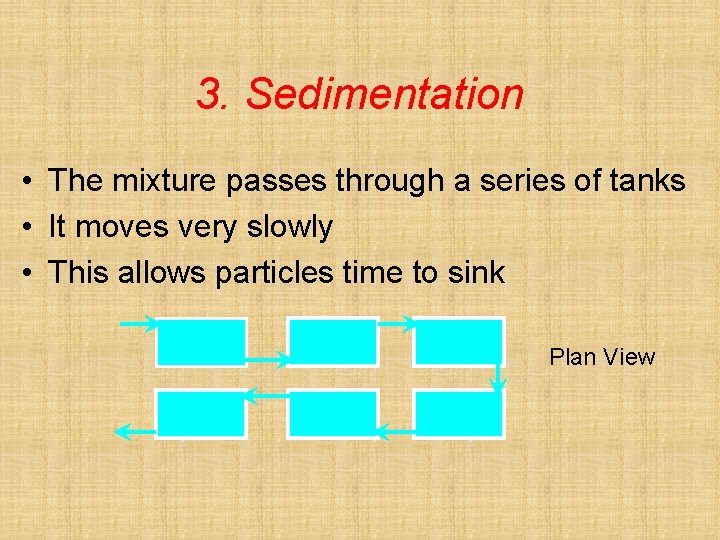
3. Sedimentation • The mixture passes through a series of tanks • It moves very slowly • This allows particles time to sink Plan View

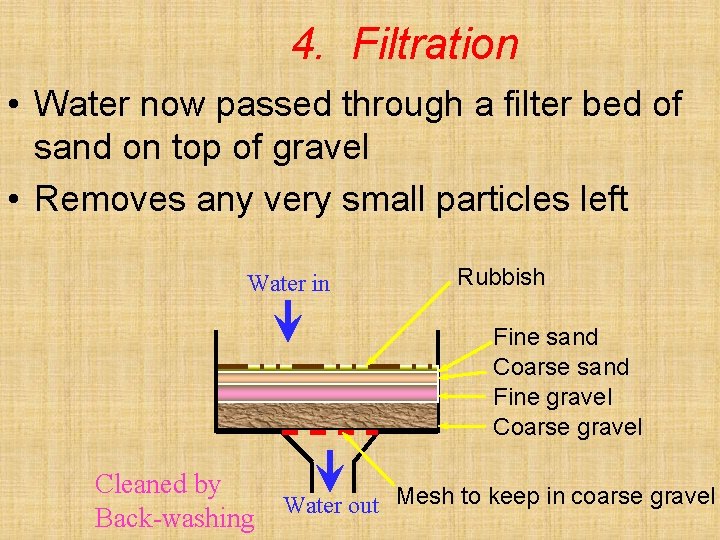
4. Filtration • Water now passed through a filter bed of sand on top of gravel • Removes any very small particles left Water in Rubbish Fine sand Coarse sand Fine gravel Coarse gravel Cleaned by Back-washing Water out Mesh to keep in coarse gravel

5. Additives • Chlorine added to kill bacteria - enough to keep it safe till it reaches houses. • Fluoride added for strong teeth - no cavities • p. H balance – if water is too acid Na. OH added – if water is too alkaline H 2 SO 4 or CO 2 6. Storage • Stored underground • to prevent contamination

Sewage Treatment Needed to control disease Various degrees of treatment 1. No Treatment • Straight into rivers • Your problem washed downstream. • You get it from upstream 23

2. Primary Treatment (i) Screening – removing lumpy bits (ii) Sedimentation – let solids settle – Discharge of liquid – Composting of solids left behind kills pathogenic bacteria – Better than nothing but not great 24

3. Secondary Treatment • Follows primary and is a big improvement (i) Biological Oxidation [E. g. Activated Sludge Process] – Stir the sludge vigorously [or pour over large surface area] to oxygenate – Encourages growth of aerobic microorganisms – These digest the sewage and destroy pathogens 25

(ii) Sedimentation – Liquid then discharged into rivers etc. – Biologically safe i. e. you won’t get disease from it – Problem Eutrophication: Excess plant growth caused by excess nutrients. – When these run out the plants die, rot and use up the oxygen which kills almost all the animals and Destroys the habitat. 26

4. Tertiary Treatment • • • Follows Secondary Treatment Not common - it is expensive Removes ions by precipitation PO 43 - [phosphate] and NO 31 - [nitrate] Removal of these ions prevents eutrophication • Ions also poisonous • Water now completely safe. 27
![Eutrophication Excess plant growth algae caused by excess nutrients in water Mainly caused Eutrophication Excess plant growth [algae] caused by excess nutrients in water. • Mainly caused](https://slidetodoc.com/presentation_image_h/0d0bc22b9b7c75cbdefb89a5eedb3cd8/image-28.jpg)
Eutrophication Excess plant growth [algae] caused by excess nutrients in water. • Mainly caused by excess nitrates [NO 31 -] and phosphates[PO 43 -] Sources of nutrients – Fertilisers washed off land – Silage effluent – Pig slurry – Sewage etc. 28

Results of Eutrophication • • • When nutrients run out Cannot sustain plant life. [algae] Plants [algae] die Bacteria rot plant remains Aerobic bacteria use up oxygen turn place anaerobic • Then anaerobic bacteria take over producing H 2 S which sours [poisons] the environment • Only a few specialised animals can survive • Death of pond, river etc. 29

Other Concerns • High Nitrate content in water may cause – Stomach cancer – Death in babies [when powdered milk and nitrate rich water is used to make their food] 30

Heavy Metal Pollution

Introduction • • • Release of toxic metal ions into water Lead Pb 2+, mercury Hg 2+, cadmium Cd 2+ Ions Called Heavy Metals due to high RAM Cumulative poisons Concentrations build up in body on continuous exposure [over time] Sources (i) Industrial effluent (ii) From batteries of various kinds if not recycled (iii) Pb 2+ from the water pipes of old houses - especially if water is acid

Case Study • • • Mercury metal Poisonous inhaled into lungs Not poisonous if swallowed Passes out in a few days Inorganic mercury salts very dangerous at high concentrations. E. g. Hg. Cl 2, Hg(NO 3)2 etc. • Organic at low levels • Damage kidneys and intestine • Makes one “poco loco”. “Mad as a Hatter”

• • Minimata Bay Japan - Late 1950’s Industrial waste discharged into bay Hg salts got into food chain High concentrations built up in fish People ate a lot of fish Caused birth defects and death Called Minimata Disease
![E U Limits For levels of nitrate phosphate and specific metal ions 2 examples E U Limits For levels of nitrate, phosphate and specific metal ions [2 examples]](https://slidetodoc.com/presentation_image_h/0d0bc22b9b7c75cbdefb89a5eedb3cd8/image-35.jpg)
E U Limits For levels of nitrate, phosphate and specific metal ions [2 examples] (aq) Maximum admissible Substance : Concentration Nitrates : 50 mg / L Phosphates : 2. 2 mg / L Lead : 50 mg / L Mercury : 1 µg / L

Removal Usually removed before discharge (i) Removed by precipitation Pb 2+(aq) + 2 Cl-(aq) = Pb. Cl 2(s) (ii) Ion exchange

Water Analysis Instrumental Methods

p. H Meter • Used to check river and lake water • Use Buffer Solutions* to calibrate p. H meter [*keeps p. H constant] • Monitors p. H constantly • Adjust p. H of water to keep it within limits 7 -9

Colorimeter • White light passed through a coloured solution • The colour of the solution is then compared to the colour of solutions of known concentration

Principle on which it works Colour Intensity is directly proportional to concentration Social and Applied Aspects • Fertilisers in water • Chlorine in pools

Atomic Absorption Spectrometer (AAS) Principles • Ground state atoms of an element absorb light characteristic of that element • Absorbance is directly proportional to concentration

Processes • • Dissolving of sample Sample introduction (as a fine spray) Atomisation of sample (in a flame) Absorption (by the sample) of light of characteristic wavelength by the specific element • Detection – amount of light absorbed depends on amount of element in sample

Atomic Absorption Spectrometer • Used to detect and measure the concentration of Heavy Metals 1. Lead Pb 2+ in water and blood 2. Cadmium Cd 2+ 3. Mercury 2+ Hg

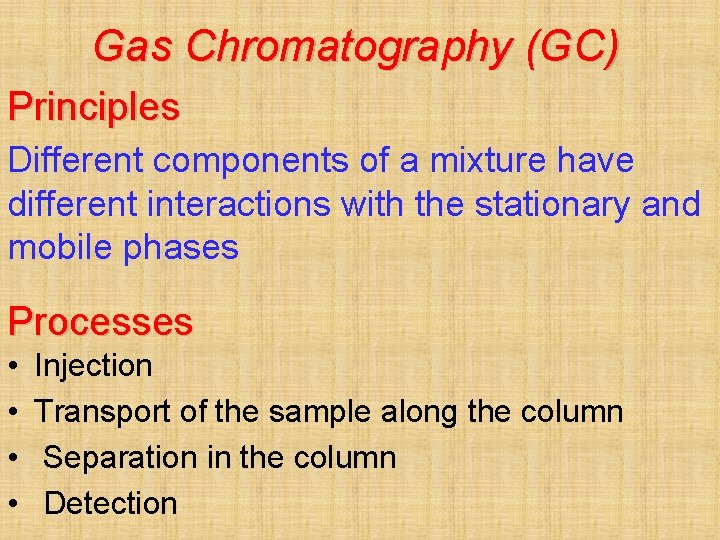
Gas Chromatography (GC) Principles Different components of a mixture have different interactions with the stationary and mobile phases Processes • • Injection Transport of the sample along the column Separation in the column Detection

Gas Chromatography

Gas Chromatography (GC) • Mobile phase = a gas • Stationary phase = non-volatile liquid coated on fine particles of an inert solid • Used to separate more volatile mixtures Social and Applied Aspects • Drug tests on athletes • Blood alcohol tests

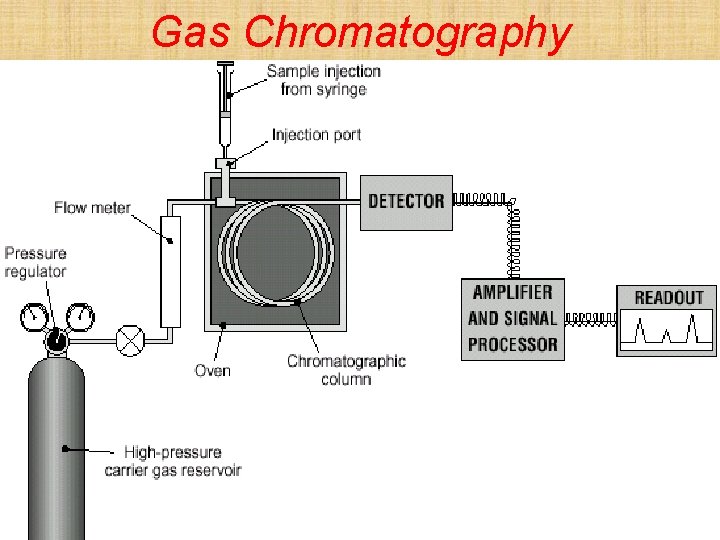
High Performance Liquid Chromatography (HPLC) Principle • Different components of a mixture have different tendencies to adsorb onto very fine particles of a solid in the HPLC column • Injection • Transport of the sample along the column • Separation in the column • Detection • Mobile phase = a solvent • Stationary phase = very fine particles of silica • Used to separate less volatile mixtures

Social and Applied Aspects • Analysis of growth promoters in meat • Analysis of vitamins in food

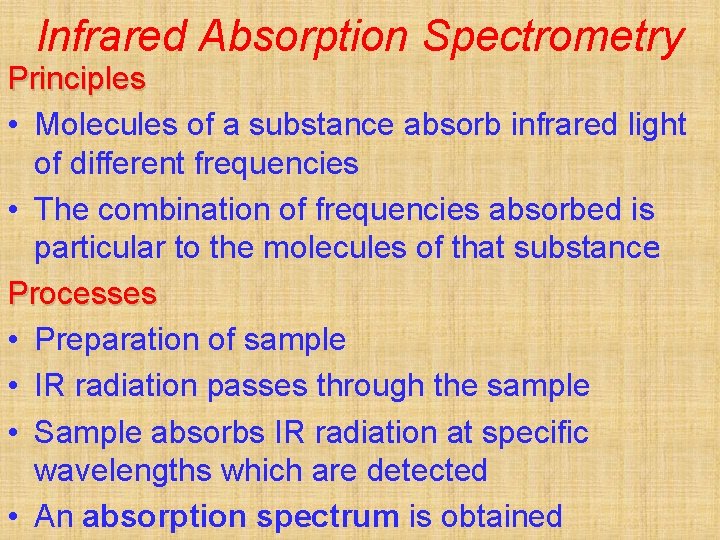
Infrared Absorption Spectrometry Principles • Molecules of a substance absorb infrared light of different frequencies • The combination of frequencies absorbed is particular to the molecules of that substance Processes • Preparation of sample • IR radiation passes through the sample • Sample absorbs IR radiation at specific wavelengths which are detected • An absorption spectrum is obtained

Social and Applied Aspects • Identification of plastics • Identification of drugs

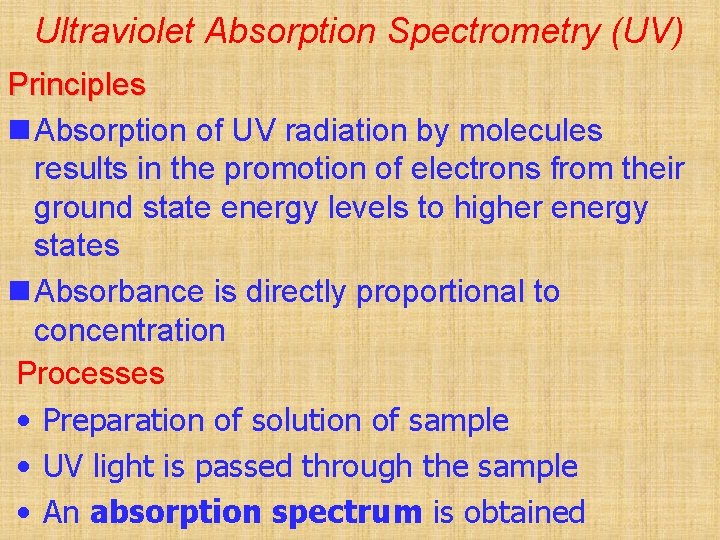
Ultraviolet Absorption Spectrometry (UV) Principles n Absorption of UV radiation by molecules results in the promotion of electrons from their ground state energy levels to higher energy states n Absorbance is directly proportional to concentration Processes • Preparation of solution of sample • UV light is passed through the sample • An absorption spectrum is obtained

Social and Applied Aspects • Quantitative determination of drug metabolites • Quantitative determination of plant pigments

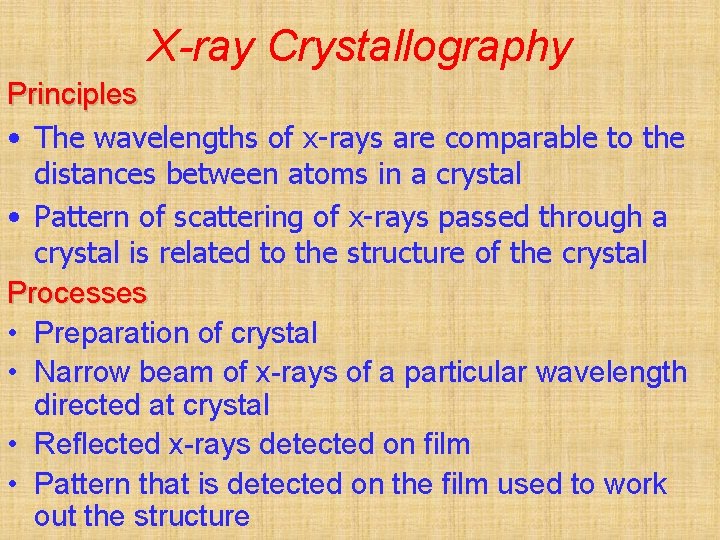
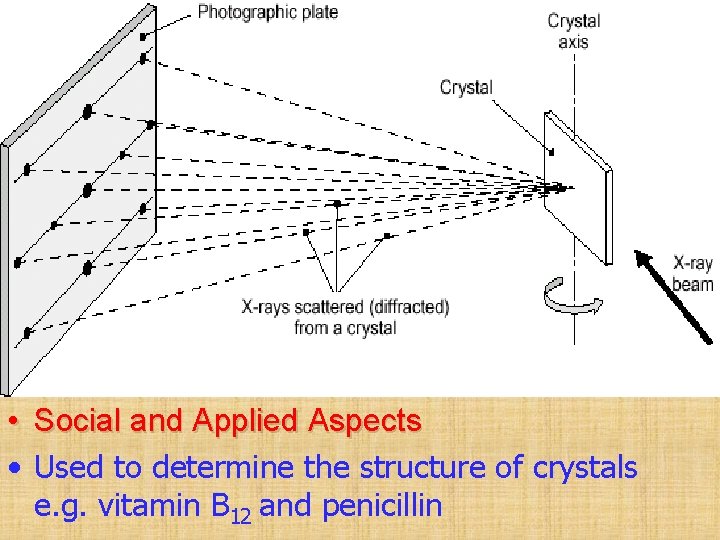
X-ray Crystallography Principles • The wavelengths of x-rays are comparable to the distances between atoms in a crystal • Pattern of scattering of x-rays passed through a crystal is related to the structure of the crystal Processes • Preparation of crystal • Narrow beam of x-rays of a particular wavelength directed at crystal • Reflected x-rays detected on film • Pattern that is detected on the film used to work out the structure

• Social and Applied Aspects • Used to determine the structure of crystals e. g. vitamin B 12 and penicillin
 Gravimetric analysis of calcium and hard water
Gravimetric analysis of calcium and hard water Discuss how permanent hardness of water can be removed
Discuss how permanent hardness of water can be removed How to calculate total hardness
How to calculate total hardness Water hardness equation
Water hardness equation Define hardness of water
Define hardness of water Cara screenshot laptop
Cara screenshot laptop What is the definition of sanitizing servsafe
What is the definition of sanitizing servsafe Removal of water hardness
Removal of water hardness Pseudo hardness
Pseudo hardness Units of hardness of water
Units of hardness of water Work hard have fun make history
Work hard have fun make history Hard times hard drive
Hard times hard drive Some any cheese
Some any cheese Any to any connectivity
Any to any connectivity Pertanyaan terbuka
Pertanyaan terbuka Water and water and water water
Water and water and water water Lecture table minecraft
Lecture table minecraft Gypsum hardness
Gypsum hardness Workshop test for toughness
Workshop test for toughness Hardness test is the ability of a material to resist
Hardness test is the ability of a material to resist Hsab principle
Hsab principle Mica mineral hardness
Mica mineral hardness Total hardness calculation
Total hardness calculation Notching hair
Notching hair Erection hardness scale
Erection hardness scale Silicate hardness
Silicate hardness Study of minerals
Study of minerals What is cleavage in minerals
What is cleavage in minerals Sapphire mohs hardness scale
Sapphire mohs hardness scale Mohs hardness scale drawing
Mohs hardness scale drawing Permanent hardness is also called as
Permanent hardness is also called as Properties of matter definition
Properties of matter definition Mechanical properties of nano materials
Mechanical properties of nano materials Helium physical and chemical properties
Helium physical and chemical properties Prototype hardness tester
Prototype hardness tester Tapio technologies
Tapio technologies Hardness texture color and freezing point are examples of
Hardness texture color and freezing point are examples of Properties of ionic lattices
Properties of ionic lattices Astm e 140
Astm e 140 You have a 5 and 3 gallon jug
You have a 5 and 3 gallon jug If you don't water the flowers they will die
If you don't water the flowers they will die Windows 95 max hard drive size
Windows 95 max hard drive size Soft news
Soft news Define news values
Define news values Is ib french hard
Is ib french hard Is ap chemistry hard
Is ap chemistry hard Compound adjectives examples
Compound adjectives examples Epithelium
Epithelium Soft tooling vs hard tooling
Soft tooling vs hard tooling This is a hard saying
This is a hard saying The way of a transgressor is hard
The way of a transgressor is hard Pros and cons of learning german
Pros and cons of learning german The life of a hard-working future king
The life of a hard-working future king 5 step hard style
5 step hard style What are the stages of clay
What are the stages of clay