HARDNESS OF WATER Hard water is water that









- Slides: 15

HARDNESS OF WATER

Ø Hard water is water that has high mineral content. Ø Hard water is formed of limestone and chalk which when are water percolates through largely deposits made up of calcium and magnesium carbonates. Ø Hard drinking water may have moderate health benefits, but can pose serious problems in industrial settings, where water hardness is monitored to avoid costly breakdowns in boilers, cooling towers, and other equipment that handles water. Ø In domestic settings, hard water is often indicated by a lack of leather formation when soap is agitated in water, and by the formation of limescale in kettles and water heaters.

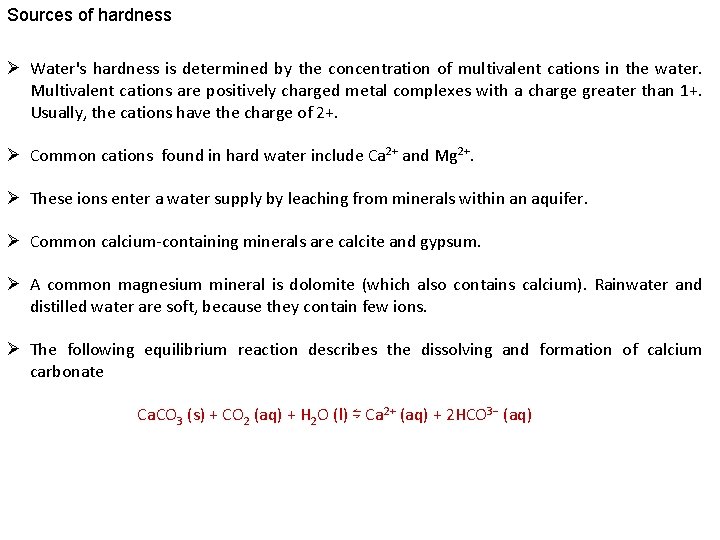
Sources of hardness Ø Water's hardness is determined by the concentration of multivalent cations in the water. Multivalent cations are positively charged metal complexes with a charge greater than 1+. Usually, the cations have the charge of 2+. Ø Common cations found in hard water include Ca 2+ and Mg 2+. Ø These ions enter a water supply by leaching from minerals within an aquifer. Ø Common calcium-containing minerals are calcite and gypsum. Ø A common magnesium mineral is dolomite (which also contains calcium). Rainwater and distilled water are soft, because they contain few ions. Ø The following equilibrium reaction describes the dissolving and formation of calcium carbonate Ca. CO 3 (s) + CO 2 (aq) + H 2 O (l) ⇋ Ca 2+ (aq) + 2 HCO 3− (aq)






Hard water and soap Rainwater becomes slightly acidic as carbon dioxide from the air dissolves in it, forming carbonic acid. Limestone contains calcium carbonate. As the slightly acidic rainwater trickles through rocks, the calcium carbonate reacts to form soluble calcium hydrogen carbonate: carbonic acid + calcium carbon calcium hydrogen + + water carbonate dioxide carbonate H 2 CO 3 (aq) + Ca. CO 3 (s) Ca(HCO 3)2 (aq) + CO 2 (g) + H 2 O (l) One of the chemicals in soap is sodium stearate. The dissolved calcium and magnesium compounds in hard water react with sodium stearate to form a solid called calcium stearate (or ‘scum’). 9 of 55 © Boardworks Ltd 2009

How does hard water react with soap? Soap ‘scum’ is formed by the reaction: sodium stearate + calcium hydrogen carbonate calcium stearate + sodium hydrogen carbonate 2 C 17 H 35 COONa (s) + Ca(HCO 3)2 (aq) (C 17 H 35 COO)2 Ca (s) + 2 Na. HCO 3 (aq) The soap will only form a lather when all the dissolved calcium hydrogen carbonate in the water has reacted. Using hard water can cause problems: l More soap is needed to get a lather. l It can be difficult to clean the scum from bathtubs and sinks. l Hard water can be unsuitable for industrial processes like dying. 10 of 55 © Boardworks Ltd 2009

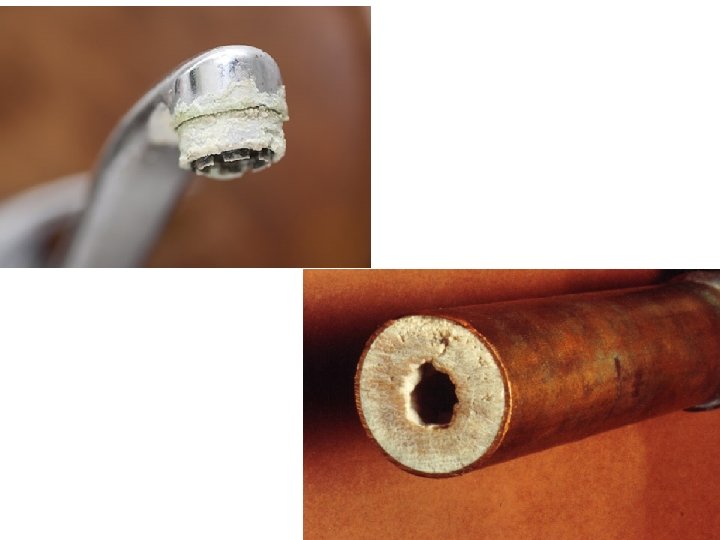
Limescale When hard water is heated, the dissolved calcium hydrogen carbonate decomposes to form solid calcium carbonate. These deposits of calcium carbonate are called limescale. calcium hydrogen calcium carbonate + water Ca. CO 3 (s) + H 2 O (l) + Ca(HCO 3)2 (aq) + carbon dioxide CO 2 (g) Limescale can block pipes and coat the heating elements in kettles, washing machines and heaters. Limescale is a poor heat conductor, and reduces the efficiency of appliances. 11 of 55 © Boardworks Ltd 2009

Removing limescale Weak acids, such as ethanoic acid, can be used as descalers. The acid reacts with the limescale to form soluble compounds, which are then washed away. ethanoic + acid calcium carbonate calcium ethanoate + carbon + water dioxide 2 CH 3 COOH (aq) + Ca. CO 3 (s) (CH 3 COO)2 Ca (aq) + CO 2 (g) + H 2 O (l) What would a person observing this reaction see? 12 of 55 © Boardworks Ltd 2009

What are the benefits of hard water? Hard water can be good for health: l calcium is needed for healthy bones and teeth l magnesium is needed for effective metabolism. Some studies have also shown that people living in hard water areas are less likely to suffer from heart disease. The World Health Organisation states that there is not yet enough evidence to confirm a link between hard water and heart disease. What evidence would you gather to look for a link between hard water and heart disease? 13 of 55 © Boardworks Ltd 2009

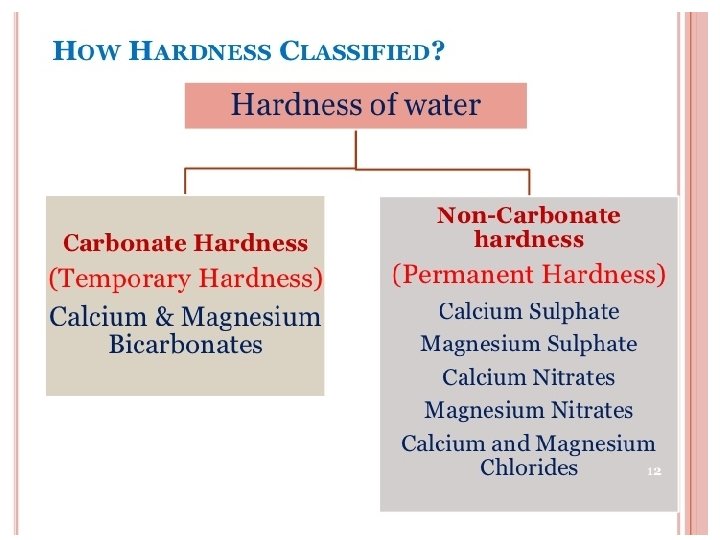
Softening temporary hard water Temporary hard water contains calcium hydrogen carbonate. This is relatively easy to remove, because it decomposes on heating to form solid calcium carbonate: calcium hydrogen calcium carbonate + water Ca. CO 3 (s) + H 2 O (l) + Ca(HCO 3)2 (aq) + carbon dioxide CO 2 (g) Knowing this, how would you remove the solid calcium carbonate from the water? What would be left behind after removing temporary hardness using this method? 14 of 55 © Boardworks Ltd 2009

Softening hard water Both temporary and permanent hard water can be softened by adding sodium carbonate (washing soda). The sodium carbonate reacts with the calcium compounds in the water to form calcium carbonate and soluble sodium compounds, which do not contribute to hardness: calcium hydrogen carbonate + sodium carbonate Ca(HCO 3)2 (aq) + Na 2 CO 3 (s) sodium hydrogen carbonate calcium carbonate + Ca. CO 3 (s) + 2 Na. HCO 3 (aq) What is the word and symbol equation for the reaction between calcium sulfate (which causes permanent hardness) and sodium carbonate? 15 of 55 © Boardworks Ltd 2009
 T.me/makeshard
T.me/makeshard Units of hardness of water
Units of hardness of water How to calculate total hardness
How to calculate total hardness Master cleaning schedule servsafe
Master cleaning schedule servsafe How to calculate total hardness
How to calculate total hardness Removal of water hardness
Removal of water hardness Define hardness of water
Define hardness of water Pseudo hardness of water
Pseudo hardness of water Discuss how permanent hardness of water can be removed
Discuss how permanent hardness of water can be removed Calcium carbonic acid
Calcium carbonic acid Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Phân độ lown
Phân độ lown Tìm vết của mặt phẳng
Tìm vết của mặt phẳng Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt





























