Hard and Soft Water Where does our drinking




















































- Slides: 67

Hard and Soft Water

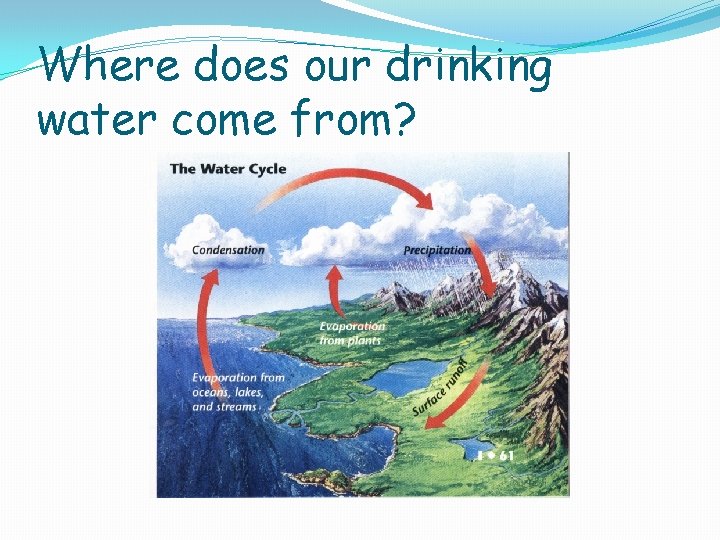
Where does our drinking water come from?

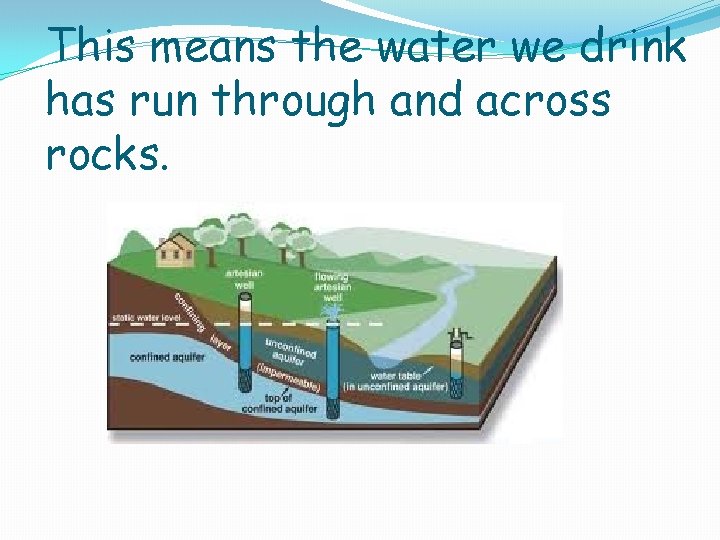
This means the water we drink has run through and across rocks.

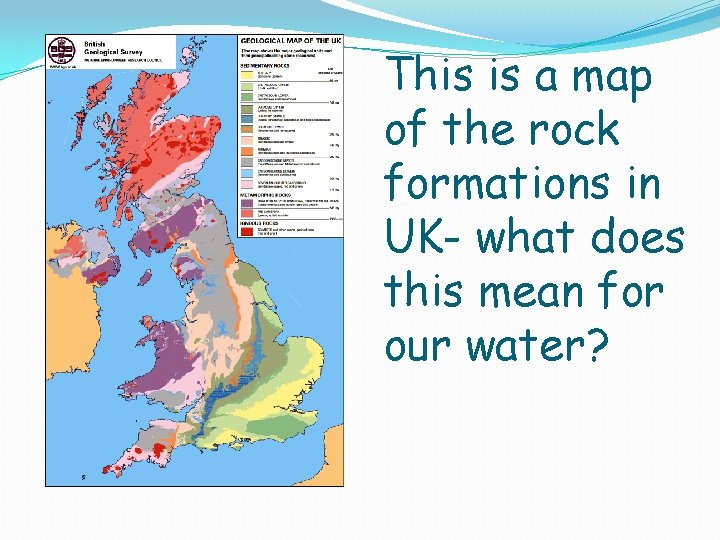
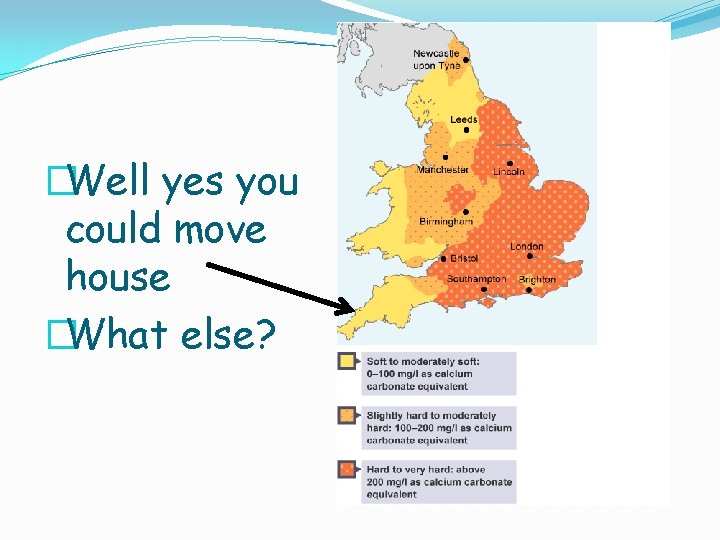
This is a map of the rock formations in UK- what does this mean for our water?

Depending where you live you will get different chemicals in your tap water. .

We call the two main water types: � Hard water � Soft water

You need to know: �The difference between hard and soft water �Which ions cause hard water �How they get there �The pros and cons of each water type �Treatments for water hardness

There are 2 metal ions we need to focus on that make water HARD �Calcium – Ca 2+ �Magnesium – Mg 2+

REMEMBER While other metal ions can be in water, only Ca 2+ and Mg 2+ cause hardness.

2+ Ca How do and into the water? 2+ Mg get You need to know the details for calcium This is a ‘limestone pavement’. Limestone is calcium carbonateit does not dissolve in pure water, BUT it does dissolve in acid rain.

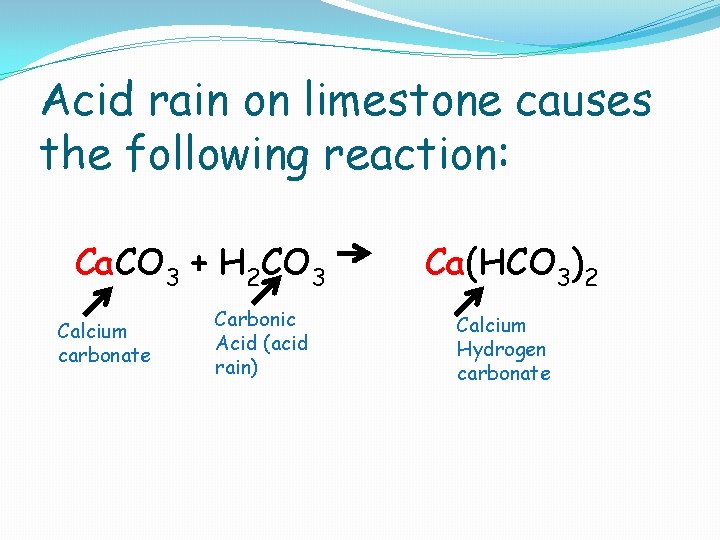
Acid rain on limestone causes the following reaction: Ca. CO 3 + H 2 CO 3 Calcium carbonate Carbonic Acid (acid rain) Ca(HCO 3)2 Calcium Hydrogen carbonate

So now you have calcium ions (and HCO 3 ions) in the water. Ca 2+

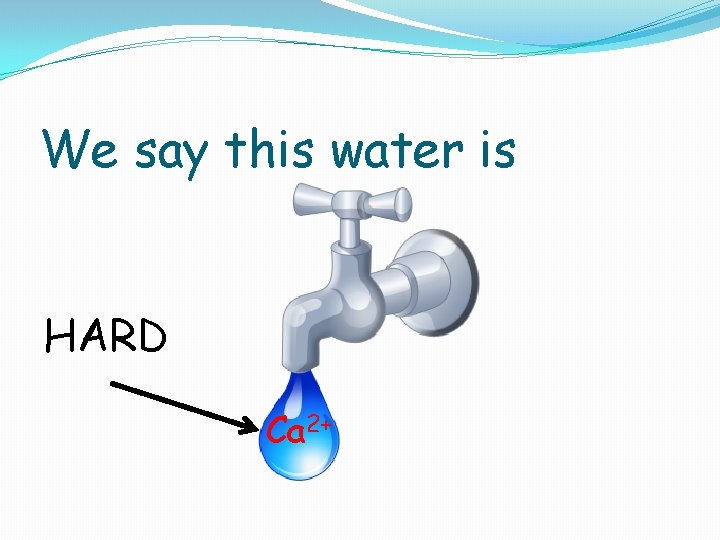
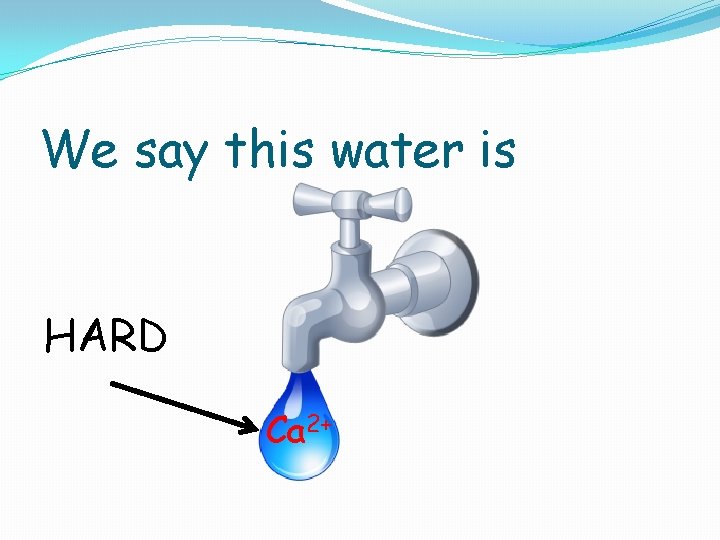
We say this water is HARD Ca 2+

These are crystals of Calcium sulphate Look at the tiny man

Unlike calcium carbonate (limestone), Calcium sulphate in rocks (Gypsum) is very soluble.

Calcium sulphate readily dissolves and puts calcium ions (and sulphate ions) in the water.

We say this water is HARD Ca 2+

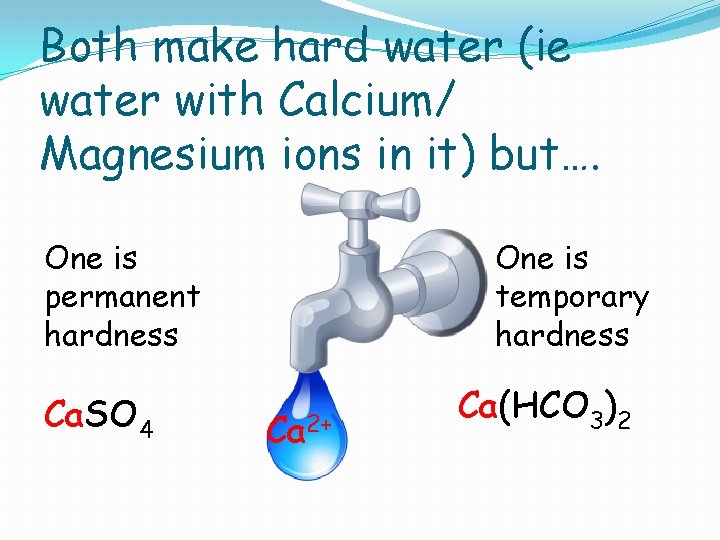
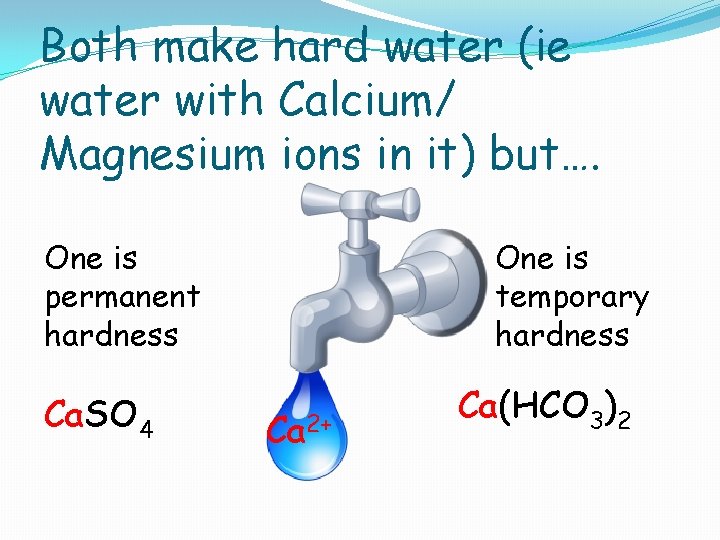
Which rocks have put the calcium in your water has important consequences: Ca. SO 4 Calcium Sulphate from gypsum OR Ca 2+ Ca(HCO 3)2 ? Calcium Hydrogen carbonate from limestone

Both make hard water (ie water with Calcium/ Magnesium ions in it) but…. One is temporary hardness One is permanent hardness Ca. SO 4 Ca 2+ Ca(HCO 3)2

Why do we care about water hardness? 3 problems: Ca 2+

1) SCUM

Calcium and magnesium ions react with soap to make calcium and magnesium stearate – or scum. �It looks horrible �It’s really hard to get off

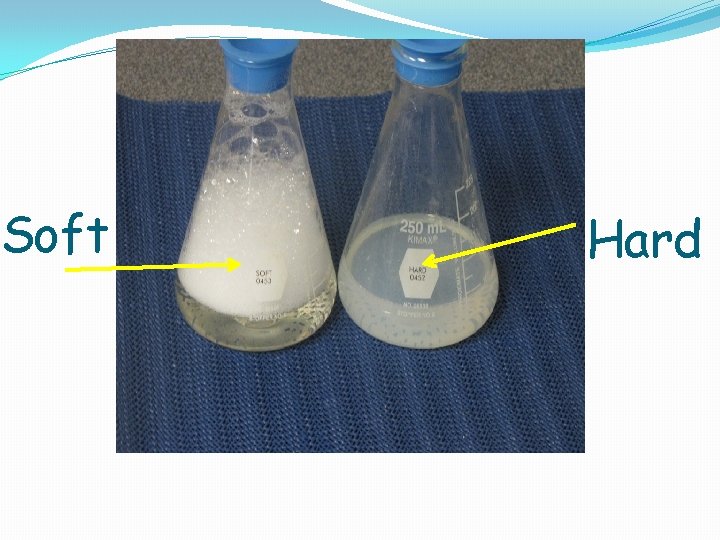
2) SOAP �Because the calcium and magnesium ions are reacting with the soap, the soap isn't doing its job ie it isn’t making bubbles.


Soft Hard

This matters in hard water areas because everyone ends up using significantly more soap.

Both permanent and temporary hardness causes those 2 problems: scum and poor bubbles.

This 3 rd problem is an issue for areas with temporary hardness, those with limestone rocks and calcium and hydrogen carbonate ions in the water.

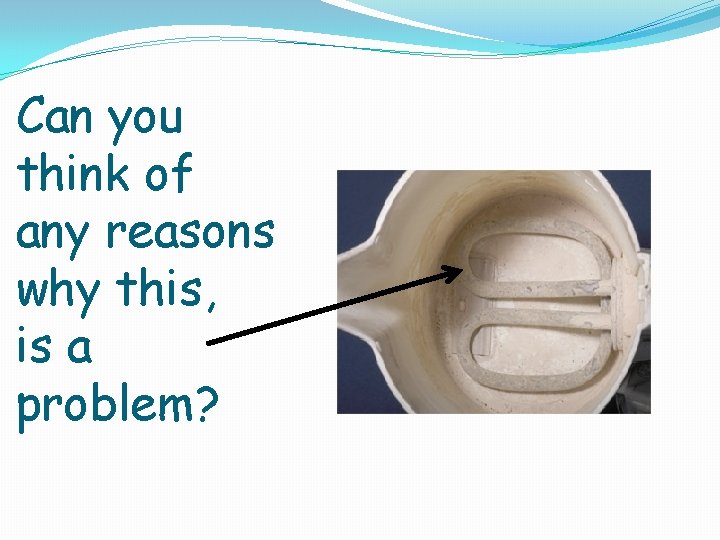
3) LIMESCALE

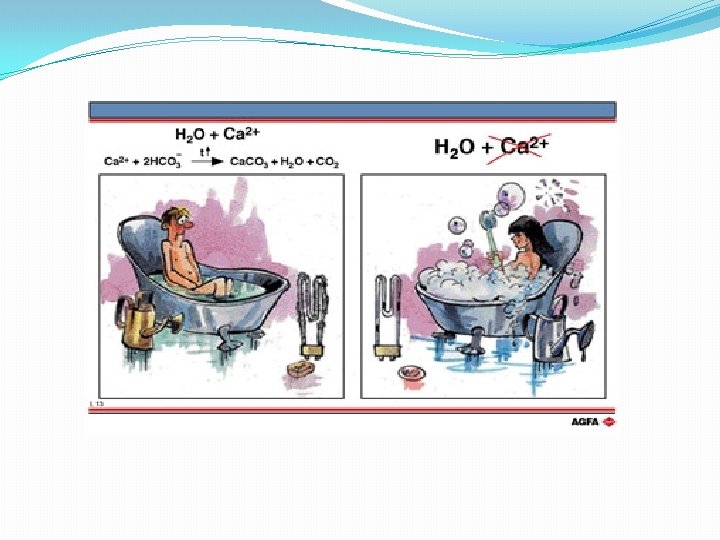
Whenever you want to heat up water which has temporary hardness, you get a problem. The hydrogen carbonate ions (HCO 3 -)from the limestone are soluble- you wont see them in the water BUT heat up these ions and they fall apart- making carbonate ions (CO 3 -) These then join up with the calcium ions to make calcium carbonate- which is NOT soluble in water (remember it only dissolved in the first place due to acid rain). This calcium carbonate precipitates out, coating the insides of boilers, kettles, pipes

Can you think of any reasons why this, is a problem?

LIMESCALE �It reduces the efficiency of any heating element, using more energy �It can block pipes and damage equipment

Are there any positives? �Living in a hard water area clearly causes issues �Some people still prefer itcan you think of any reasons why?

Pros of hard water: �Taste- some people prefer it

Pros of hard water: �It’s good for your teeth and bones (Calcium ions)

Pros of hard water: �Heart- it can protect against heart problems

If these positives of living with hard water are not enough to outweigh the negatives, what can you do?

�Well yes you could move house �What else?

THINK! �What is water hardness? �What would be the definition of removing water hardness?

To get rid of water hardness, you need to get rid of the dissolved calcium (and magnesium) ions.

We already know one way to get the calcium in temporary hard water to precipitate outcan you remember? �Heat it �The limescale that precipitates out takes the calcium ions with it- filter these off and the water it leaves behind is now soft �Problem with this heating/filtering method? �Far too expensive to heat all the water you need and filter it before using it �Even if you could afford it, this won’t work with permanently hard water.

The key is to remember its only Calcium and Magnesium ions that are the issue. If you can swap those ions for something else, you have softened the water.

There are 2 practical ways to soften water- and they work with temporary or permanently hard water.

1) Washing Soda This product is Sodium carbonate. It puts extra carbonate ions into the water They react with the calcium ions The calcium carbonate precipitates out The sodium ions are left in the water but these don't cause hardness, therefore that's no problem.

Which rocks have put the calcium in your water has important consequences: Ca. SO 4 Calcium Sulphate from gypsum OR Ca 2+ Ca(HCO 3)2 ? Calcium Hydrogen carbonate from limestone

Both make hard water (ie water with Calcium/ Magnesium ions in it) but…. One is temporary hardness One is permanent hardness Ca. SO 4 Ca 2+ Ca(HCO 3)2

Washing Soda This product is designed to reduce the soap you need Most modern washing powder has washing soda in it anyway

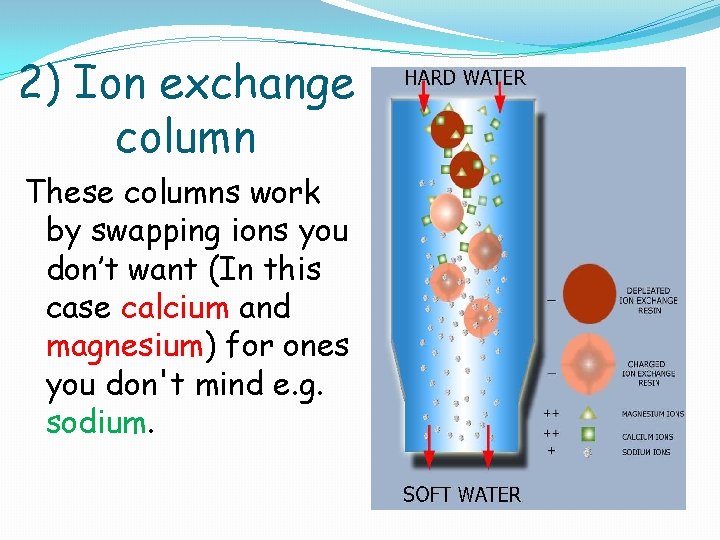
2) Ion exchange column These columns work by swapping ions you don’t want (In this case calcium and magnesium) for ones you don't mind e. g. sodium.

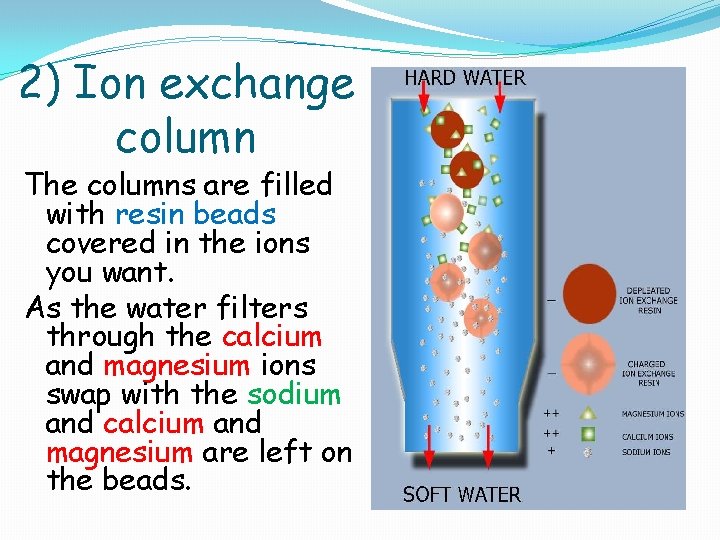
2) Ion exchange column The columns are filled with resin beads covered in the ions you want. As the water filters through the calcium and magnesium ions swap with the sodium and calcium and magnesium are left on the beads.

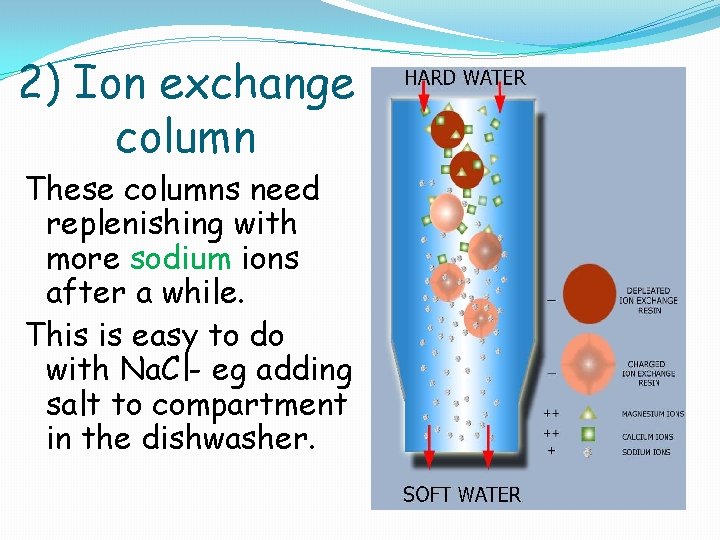
2) Ion exchange column These columns need replenishing with more sodium ions after a while. This is easy to do with Na. Cl- eg adding salt to compartment in the dishwasher.

Where do the ions in our drinking water come from? Water runs over rocks and dissolves ions

Which 2 ions cause hard water? ? Calcium and Magnesium

Which rock gives you temporary hard water? Limestone

What is the chemical name for limestone? Calcium carbonate

Why does this normally insoluble chemical dissolve? Acid rain

If you have CO 32 - ions and Calcium ions in the same water, what happens? They precipitate as Ca. CO 3

What problem arises in heating systems and temporary hard water? Limescale

Why do we care about limescale? Reduces efficiency/ blocks pipes

What rock gives you permanently hard water? Gypsum (ak. a. calcium Sulphate)

What effect does boiling have on permanently hard water? None

What do calcium and magnesium ions form with soap? Scum

What does this mean for the amount of soap needed? More soap for the same lather

What are the positives to hard water Taste/ bones and teeth/ heart

What can you add to washing water to soften it? Washing soda

How does it work? Adds carbonate ions, precipitates Ca. CO 3

What is a more sophisticated solution? Ion exchange columns

What do these columns swap with calcium / magnesium ions? ? Sodium ions