S133 Write the formula for Palladium IV Oxide















- Slides: 26

S-133 • Write the formula for – Palladium (IV) Oxide – Calcium Fluoride – Cadmium (II) Nitride Write the name for - Al 2 S 3 - Rb 3 P - Cr. O 3

Unit 7 Chemical Reaction SPS 2. Students will explore the nature of matter, its classifications, and its system for naming types of matter. d. Demonstrate the Law of Conservation of Matter in a chemical reaction. e. Apply the Law of Conservation of Matter by balancing the following types of chemical equations; 1. 2. 3. 4. Synthesis Decomposition Single Replacement Double Replacement

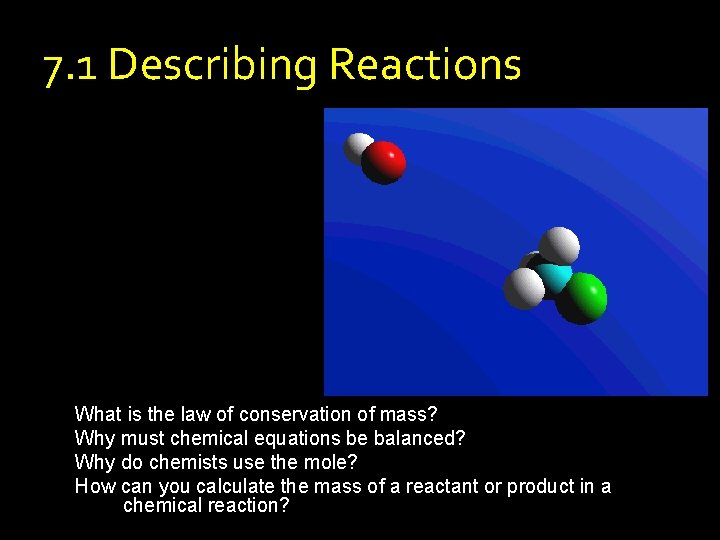
7. 1 Describing Reactions What is the law of conservation of mass? Why must chemical equations be balanced? Why do chemists use the mole? How can you calculate the mass of a reactant or product in a chemical reaction?

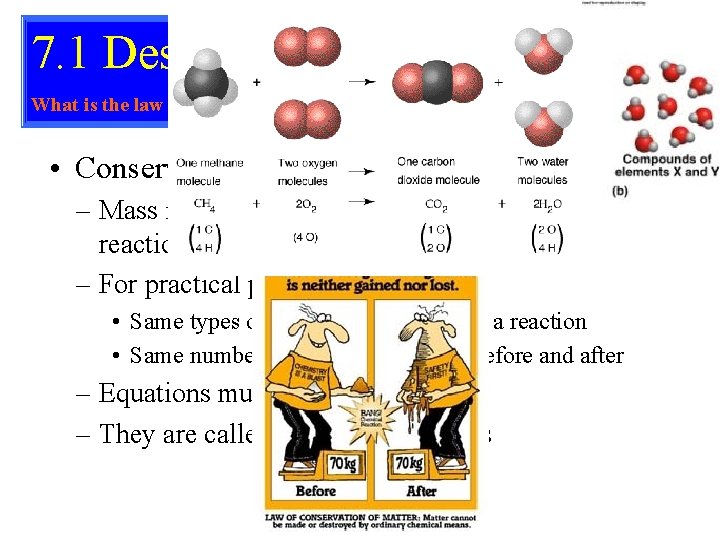
7. 1 Describing Reactions What is the law of conservation of mass? • Chemical Equations – Reactants – the substances that are present before a reaction – Products – the substances present after a reaction is complete – Always given in the form Reactants Products – Example: Carbon + Oxygen Carbon Dioxide – Or C + O 2 CO 2

7. 1 Describing Reactions What is the law of conservation of mass? • Writing Equations - Practice – Copper and Oxygen make Copper (II) Oxide – Magnesium and Hydrogen Chloride make Hydrogen and Magnesium Chloride – Ethylene (C 2 H 4) burns with Oxygen to produce Carbon Dioxide and Water. – Hydrogen and Chlorine combine to make Hydrogen Chloride

7. 1 Describing Reactions What is the law of conservation of mass? • Conservation of Mass – Mass is not created or destroyed in a chemical reaction – For practical purposes • Same types of atoms before and after a reaction • Same number of each type of atom before and after – Equations must show this – They are called balanced equations

S-134 • Write the formula for – Dinitrogen Trioxide – Copper (II) oxide – Dinitrogen Pentaoxide Write the name for - CCl 4 - Cr. Br 3 - Mo 2 O 5

S-135 • Write the equation for a reaction of hydrogen sulfide with aluminum oxide to make aluminum sulfide and water.

7. 1 Describing Reactions Why must chemical equations be balanced? • Balancing Equations – If an equation does not have the same elements on both sides, it is a false equation • Can not actually occur – Coefficient – a number placed in front of a substance in a chemical equation • Used to balance equations

7. 1 Describing Reactions Why must chemical equations be balanced? • Balancing Equations - Steps – First write out the equation • Hydrogen and Oxygen make water becomes • H 2 + O 2 H 2 O – List the elements on each side H-2 O-2 H-2 O-1 – Add substances until both sides are equal

7. 1 Describing Reactions Why must chemical equations be balanced? • Balancing Equations - Practice – Copper and Oxygen make Copper (II) Oxide – Magnesium and Hydrogen Chloride make Hydrogen and Magnesium Chloride – Ethylene (C 2 H 4) burns with Oxygen to produce Carbon Dioxide and Water.

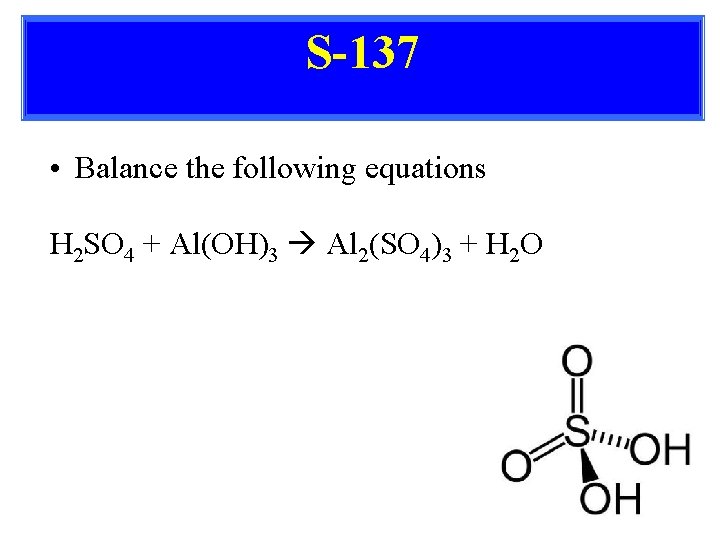
S-137 • Balance the following equations H 2 SO 4 + Al(OH)3 Al 2(SO 4)3 + H 2 O

7. 1 Describing Reactions Why do chemists use the mole? • Counting With Moles 18 Ar 39. 95 – A unit of measurement Argon 23 – Equals 6. 02 x 10 of anything – Used only to count atoms, molecules, formula unit – One mole of an element is equal to its atomic mass converted to grams (put a g beside the number)

7. 1 Describing Reactions How can you calculate the mass of a reactant or product in a reaction? • Molar Mass – For an element equal to its atomic mass – For a compound • For each element – multiply the mass x the number of that element in the compound • Add the total

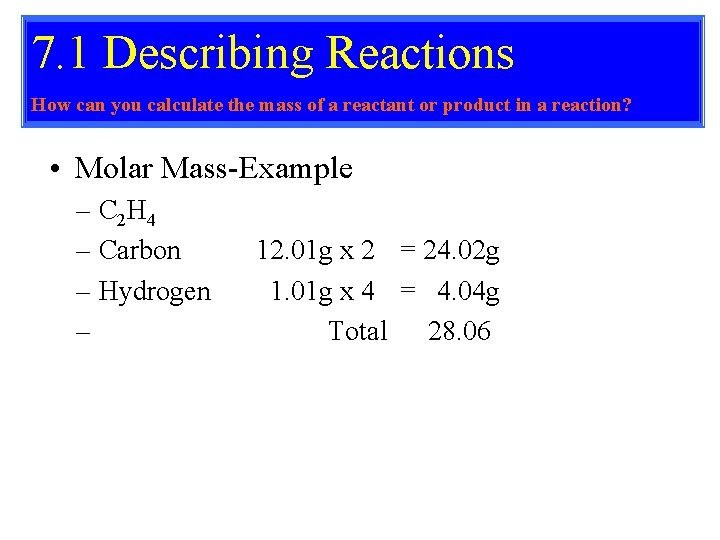
7. 1 Describing Reactions How can you calculate the mass of a reactant or product in a reaction? • Molar Mass-Example – C 2 H 4 – Carbon – Hydrogen – 12. 01 g x 2 = 24. 02 g 1. 01 g x 4 = 4. 04 g Total 28. 06

7. 1 Describing Reactions How can you calculate the mass of a reactant or product in a reaction? • Molar Mass-Example – Pb(OH)4

S-137 • What is the molar mass of Al 2(SO 4)3

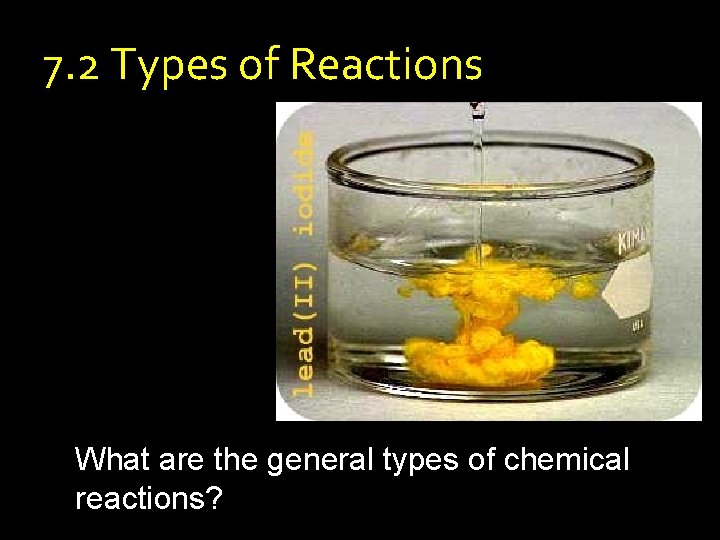
7. 2 Types of Reactions What are the general types of chemical reactions?

Types of Reactions What are the general types of reactions? • Classifying Reactions – Describe how reactants interact to form products – Help to predict the products of reactions

Types of Reactions What are the general types of reactions? • Synthesis – Two or more substances react to form a single substance – Pattern A + B AB – Always has one product – Examples • • 2 Na + Cl 2 2 Na. Cl 2 H 2 + O 2 2 H 2 O

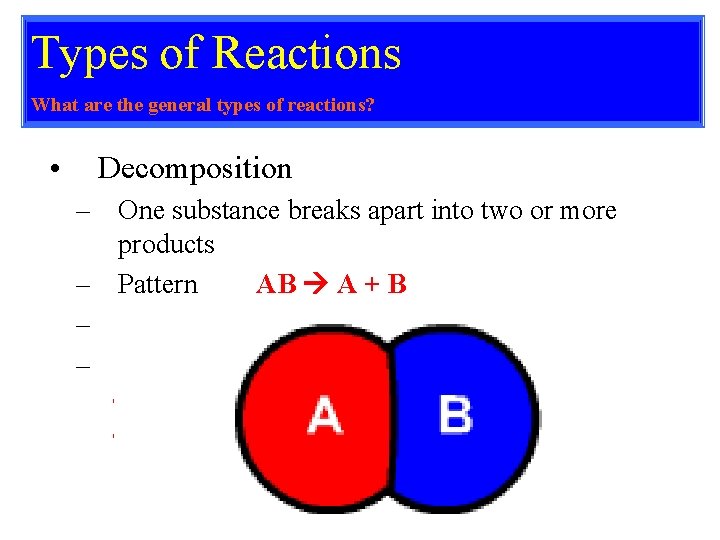
Types of Reactions What are the general types of reactions? • Decomposition – One substance breaks apart into two or more products – Pattern AB A + B – Always has one reactant – Examples • • Ca. CO 3 Ca. O + CO 2 2 H 2 O 2 H 2 + O 2

Types of Reactions What are the general types of reactions? • Single Replacement (Displacement) – A compound switches parts with an element – Pattern A + BC B + AC – Always has one element and one compound on each side – Examples • • Cu + 2 Ag. NO 3 2 Ag + Cu(NO 3)2 2 K + 2 H 2 O H 2 + KOH

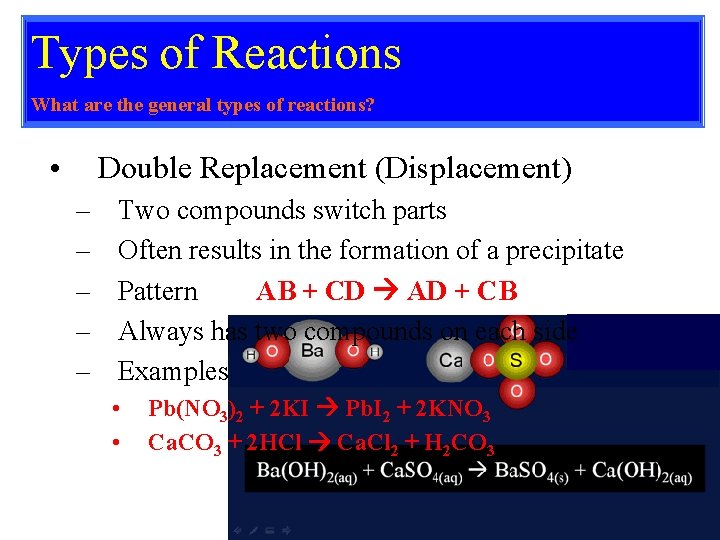
Types of Reactions What are the general types of reactions? • Double Replacement (Displacement) – – – Two compounds switch parts Often results in the formation of a precipitate Pattern AB + CD AD + CB Always has two compounds on each side Examples • • Pb(NO 3)2 + 2 KI Pb. I 2 + 2 KNO 3 Ca. CO 3 + 2 HCl Ca. Cl 2 + H 2 CO 3

Types of Reactions What are the general types of reactions? • Combustion – – – Reacts rapidly with oxygen Everything combines with oxygen Pattern AB + O 2 AO + BO Always has oxygen as a reactant Examples • • CH 4 + O 2 CO 2 + 2 H 2 O 2 Ca + O 2 2 Ca. O

S-138 • Balance the following reactions and tell what type(s) they are. 1. Pb(NO 3)2 + HCl Pb. Cl 2 + HNO 3 2. Ca + HCl Ca. Cl 2 + H 2

S-139 • Write out the reaction, then balance it and tell what type it is. 1. Mercury reacts with oxygen to form Mercury (II) Oxide