RP HPLC Purification of Small Molecules Lou Cheng




- Slides: 40

RP HPLC Purification of Small Molecules Lou Cheng Astrazeneca R&D Boston 1

Outline Ø HPLC Basics: Classification, k, α, N, Rs Ø Reversed-Phase HPLC – Isocratic and Gradient Ø Analytical Method Development – Screening & Optimization Ø Analytical Scale-up of Optimized HPLC Method Ø From Analytical Scale-up to Prep. LC Purification Ø Prep. LC Purification – Fraction Collection & Recovery Ø Summary 2

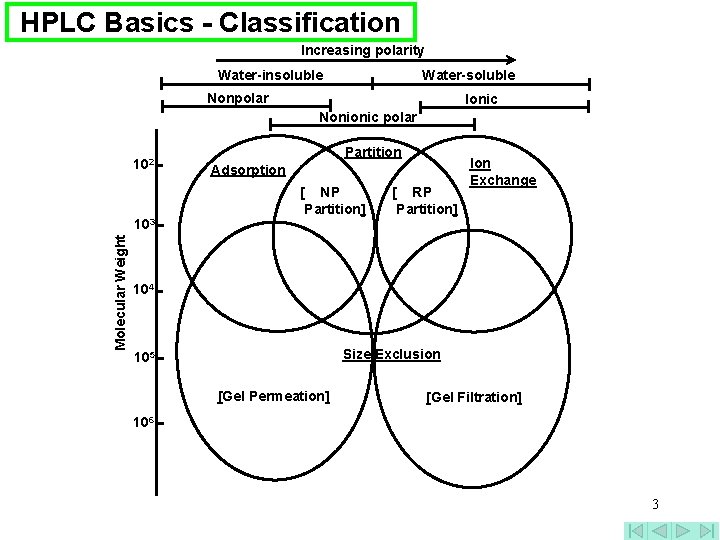
HPLC Basics - Classification Increasing polarity Water-insoluble Water-soluble Nonpolar Ionic Nonionic polar 102 Molecular Weight 103 Partition Adsorption [ NP Partition] [ RP Partition] Ion Exchange 104 Size Exclusion 105 [Gel Permeation] [Gel Filtration] 106 3

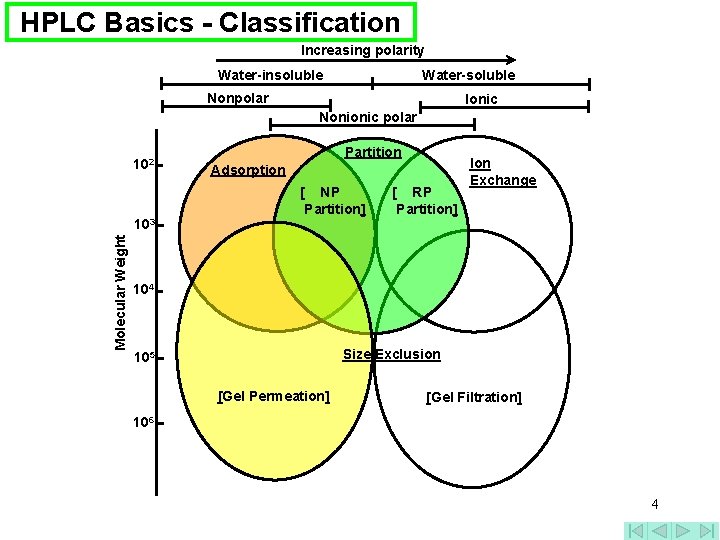
HPLC Basics - Classification Increasing polarity Water-insoluble Water-soluble Nonpolar Ionic Nonionic polar 102 Molecular Weight 103 Partition Adsorption [ NP Partition] [ RP Partition] Ion Exchange 104 Size Exclusion 105 [Gel Permeation] [Gel Filtration] 106 4

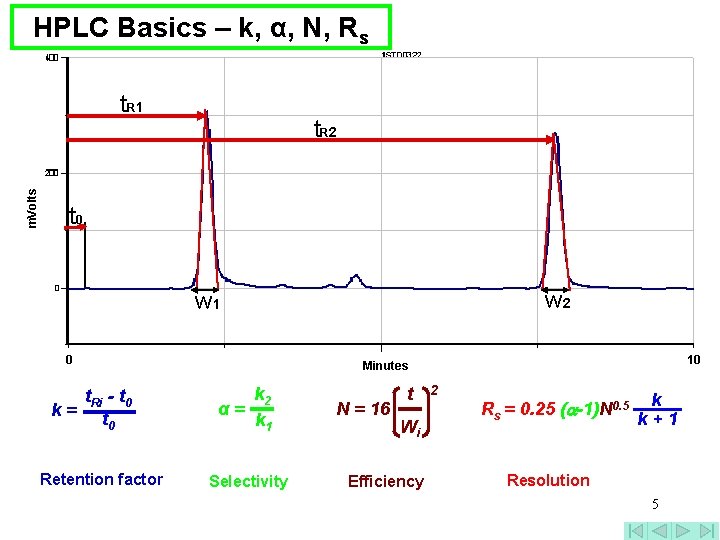
HPLC Basics – k, α, N, Rs m. Volts t. R 1 t. R 2 t 0 w 2 w 1 0 t. Ri - t 0 k= t 0 Retention factor 10 Minutes k 2 α= k 1 Selectivity N = 16 t Wi Efficiency 2 Rs = 0. 25 ( -1)N 0. 5 k k+1 Resolution 5

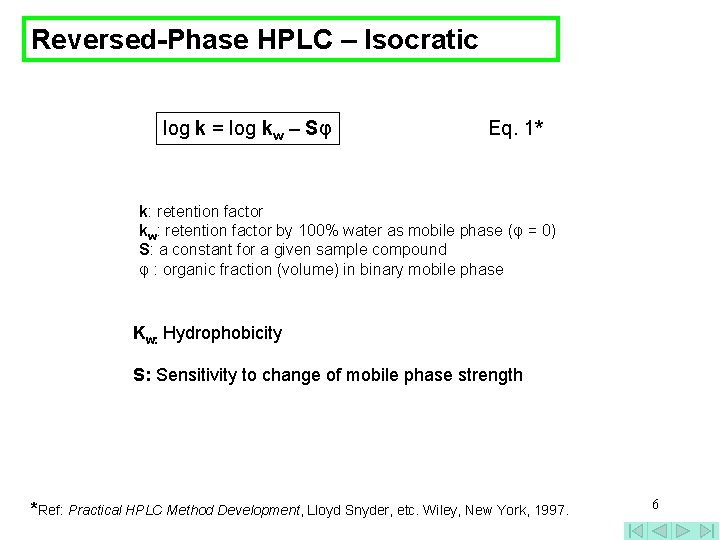
Reversed-Phase HPLC – Isocratic log k = log kw – Sφ Eq. 1* k: retention factor kw: retention factor by 100% water as mobile phase (φ = 0) S: a constant for a given sample compound φ : organic fraction (volume) in binary mobile phase Kw: Hydrophobicity S: Sensitivity to change of mobile phase strength *Ref: Practical HPLC Method Development, Lloyd Snyder, etc. Wiley, New York, 1997. 6

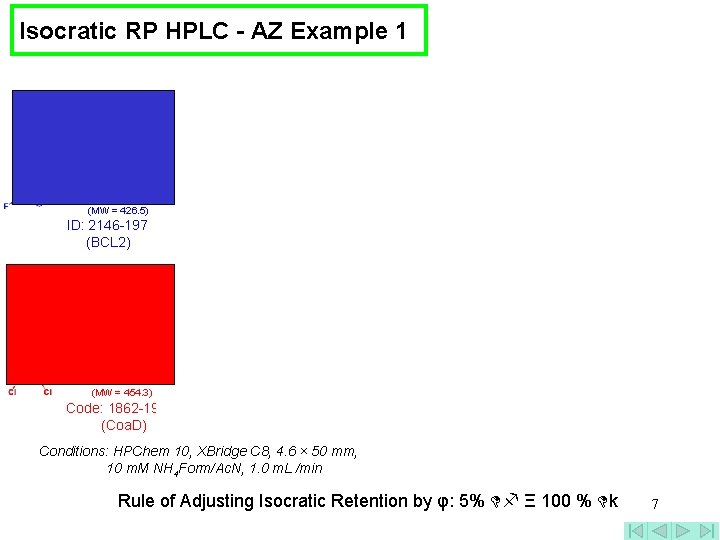
Isocratic RP HPLC - AZ Example 1 Log k = log kw – Sφ (MW = 426. 5) ID: 2146 -197 (BCL 2) (MW = 454. 3) Code: 1862 -191 -1 (Coa. D) Conditions: HPChem 10, XBridge C 8, 4. 6 × 50 mm, 10 m. M NH 4 Form/Ac. N, 1. 0 m. L /min Rule of Adjusting Isocratic Retention by φ: 5% Ξ 100 % k 7

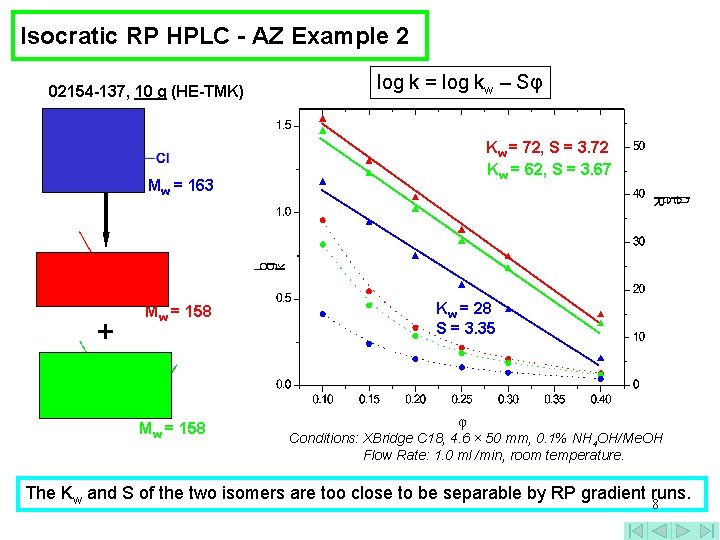
Isocratic RP HPLC - AZ Example 2 02154 -137, 10 g (HE-TMK) Mw = 163 + Mw = 158 log k = log kw – Sφ Kw = 72, S = 3. 72 Kw = 62, S = 3. 67 Kw = 28 S = 3. 35 Conditions: XBridge C 18, 4. 6 × 50 mm, 0. 1% NH 4 OH/Me. OH Flow Rate: 1. 0 ml /min, room temperature. The Kw and S of the two isomers are too close to be separable by RP gradient runs. 8

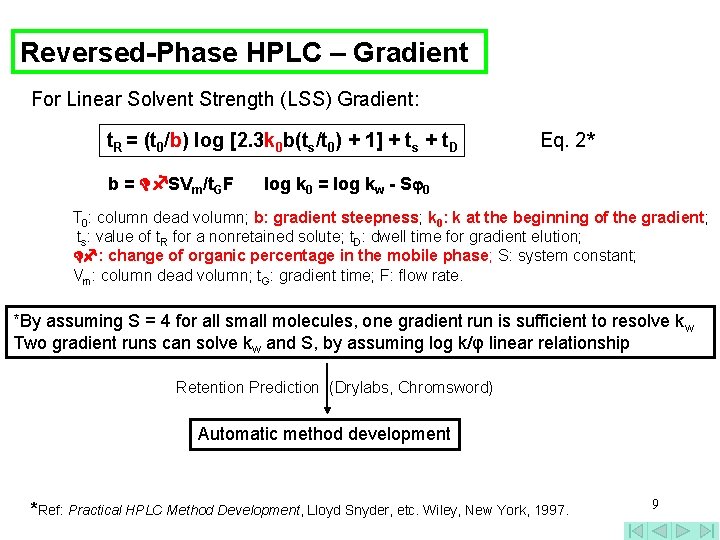
Reversed-Phase HPLC – Gradient For Linear Solvent Strength (LSS) Gradient: t. R = (t 0/b) log [2. 3 k 0 b(ts/t 0) + 1] + ts + t. D b = SVm/t. GF Eq. 2* log k 0 = log kw - S 0 T 0: column dead volumn; b: gradient steepness; k 0: k at the beginning of the gradient; ts: value of t. R for a nonretained solute; t. D: dwell time for gradient elution; : change of organic percentage in the mobile phase; S: system constant; Vm: column dead volumn; t. G: gradient time; F: flow rate. *By assuming S = 4 for all small molecules, one gradient run is sufficient to resolve kw Two gradient runs can solve kw and S, by assuming log k/φ linear relationship Retention Prediction (Drylabs, Chromsword) Automatic method development *Ref: Practical HPLC Method Development, Lloyd Snyder, etc. Wiley, New York, 1997. 9

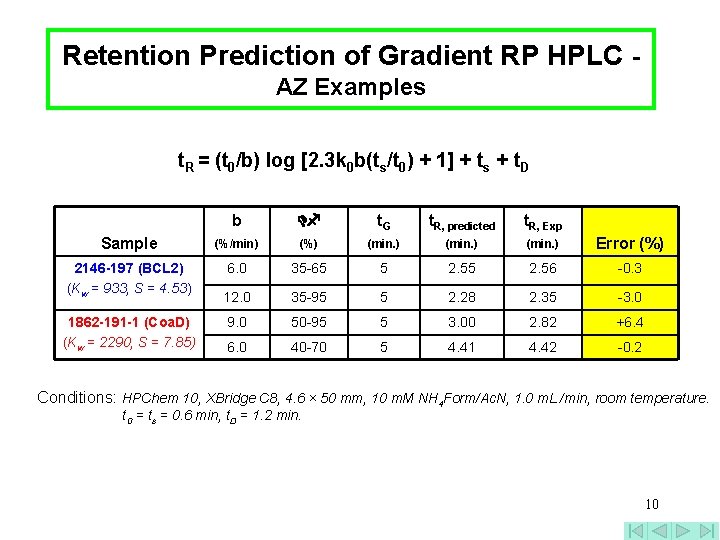
Retention Prediction of Gradient RP HPLC AZ Examples t. R = (t 0/b) log [2. 3 k 0 b(ts/t 0) + 1] + ts + t. D b t. G t. R, predicted t. R, Exp Sample (%/min) (%) (min. ) Error (%) 2146 -197 (BCL 2) (Kw = 933, S = 4. 53) 6. 0 35 -65 5 2. 56 -0. 3 12. 0 35 -95 5 2. 28 2. 35 -3. 0 9. 0 50 -95 5 3. 00 2. 82 +6. 4 6. 0 40 -70 5 4. 41 4. 42 -0. 2 1862 -191 -1 (Coa. D) (Kw = 2290, S = 7. 85) Conditions: HPChem 10, XBridge C 8, 4. 6 × 50 mm, 10 m. M NH 4 Form/Ac. N, 1. 0 m. L /min, room temperature. t 0 = ts = 0. 6 min, t. D = 1. 2 min. 10

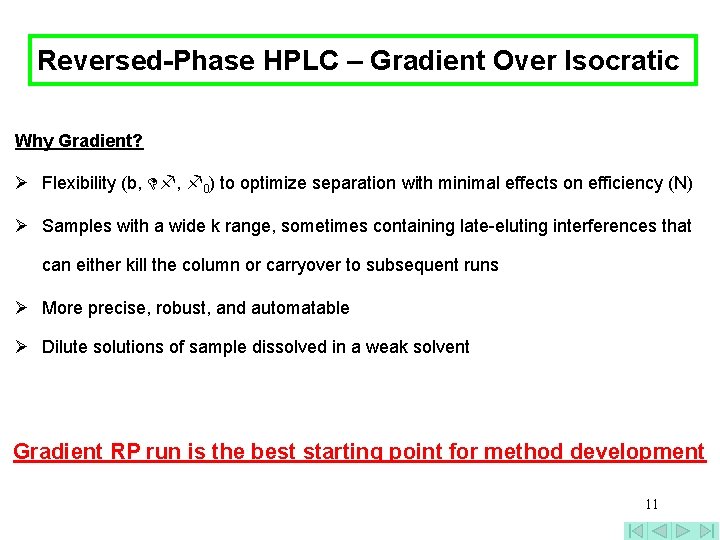
Reversed-Phase HPLC – Gradient Over Isocratic Why Gradient? Ø Flexibility (b, , 0) to optimize separation with minimal effects on efficiency (N) Ø Samples with a wide k range, sometimes containing late-eluting interferences that can either kill the column or carryover to subsequent runs Ø More precise, robust, and automatable Ø Dilute solutions of sample dissolved in a weak solvent Gradient RP run is the best starting point for method development 11

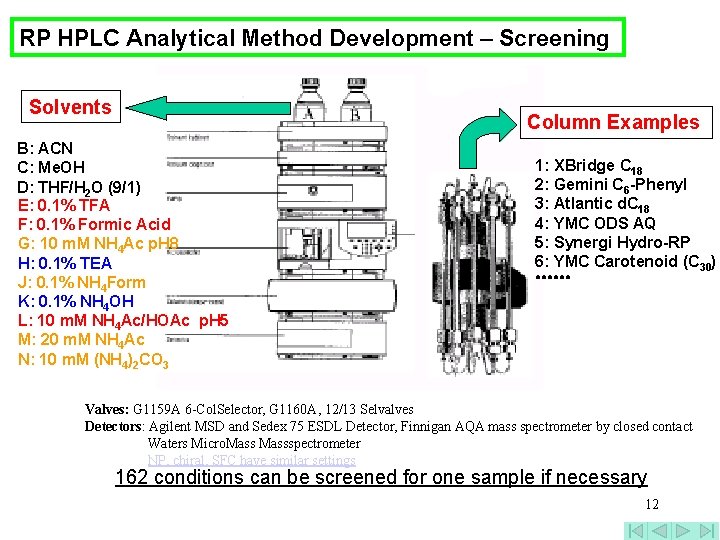
RP HPLC Analytical Method Development – Screening Solvents Column Examples B: ACN C: Me. OH D: THF/H 2 O (9/1) E: 0. 1% TFA F: 0. 1% Formic Acid G: 10 m. M NH 4 Ac p. H 8 H: 0. 1% TEA J: 0. 1% NH 4 Form K: 0. 1% NH 4 OH L: 10 m. M NH 4 Ac/HOAc p. H 5 M: 20 m. M NH 4 Ac N: 10 m. M (NH 4)2 CO 3 1: XBridge C 18 2: Gemini C 6 -Phenyl 3: Atlantic d. C 18 4: YMC ODS AQ 5: Synergi Hydro-RP 6: YMC Carotenoid (C 30) ●●●●●● Valves: G 1159 A 6 -Col. Selector, G 1160 A, 12/13 Selvalves Detectors: Agilent MSD and Sedex 75 ESDL Detector, Finnigan AQA mass spectrometer by closed contact Waters Micro. Massspectrometer NP, chiral, SFC have similar settings 162 conditions can be screened for one sample if necessary 12

Criteria for Evaluating and Optimizing HPLC Methods General: • • Low k, low tailing factor, high N High α, high Rs Client-specific: • Fraction collection for one component, multiple components, or all components? • Purity/Recovery? • p. H stability of the desired components? • MPS/library (universal applicability) • Amount of the sample (high loading) ●●●●●● Clear communication with clients is a prerequisite to successful method development 13

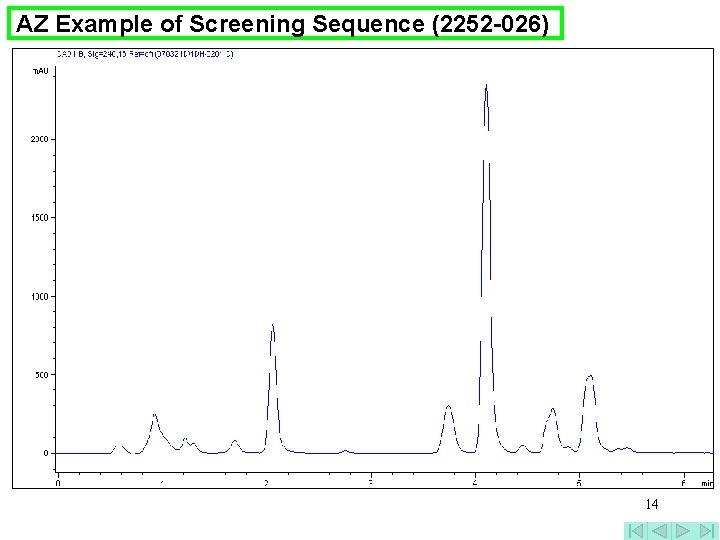
AZ Example of Screening Sequence (2252 -026) 14

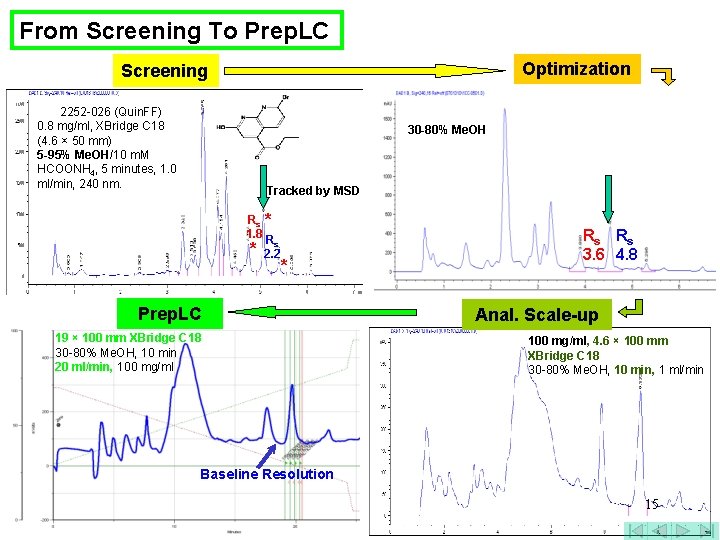
From Screening To Prep. LC Optimization Screening 2252 -026 (Quin. FF) 0. 8 mg/ml, XBridge C 18 (4. 6 × 50 mm) 5 -95% Me. OH/10 m. M HCOONH 4, 5 minutes, 1. 0 ml/min, 240 nm. 30 -80% Me. OH Tracked by MSD Rs * 1. 8 R * s 2. 2 * Prep. LC 19 × 100 mm XBridge C 18 30 -80% Me. OH, 10 min 20 ml/min, 100 mg/ml Rs Rs 3. 6 4. 8 Anal. Scale-up 100 mg/ml, 4. 6 × 100 mm XBridge C 18 30 -80% Me. OH, 10 min, 1 ml/min Baseline Resolution 15

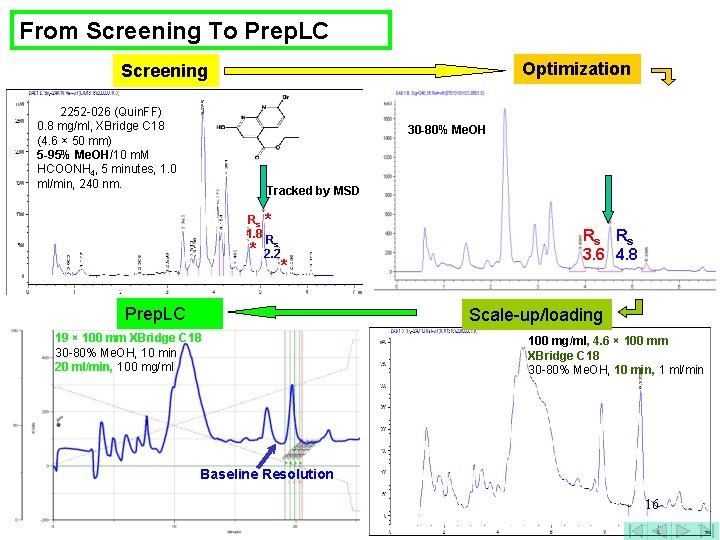
From Screening To Prep. LC Optimization Screening 2252 -026 (Quin. FF) 0. 8 mg/ml, XBridge C 18 (4. 6 × 50 mm) 5 -95% Me. OH/10 m. M HCOONH 4, 5 minutes, 1. 0 ml/min, 240 nm. 30 -80% Me. OH Tracked by MSD Rs * 1. 8 R * s 2. 2 * Prep. LC Rs Rs 3. 6 4. 8 Scale-up/loading 19 × 100 mm XBridge C 18 30 -80% Me. OH, 10 min 20 ml/min, 100 mg/ml, 4. 6 × 100 mm XBridge C 18 30 -80% Me. OH, 10 min, 1 ml/min Baseline Resolution 16

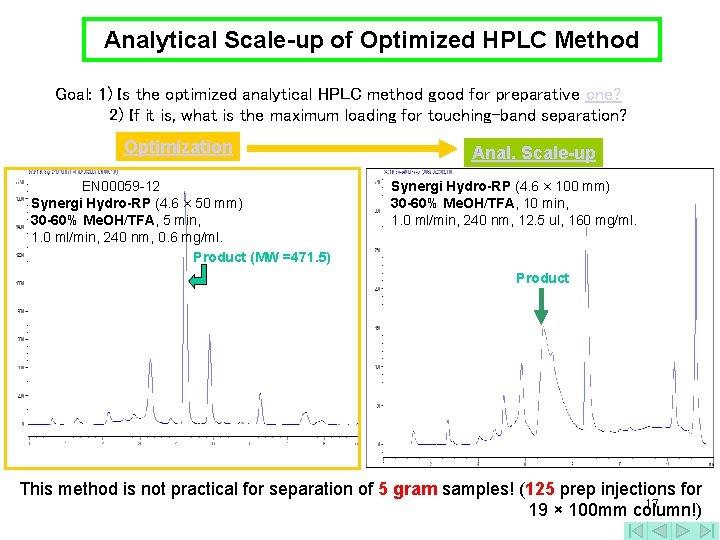
Analytical Scale-up of Optimized HPLC Method Goal: 1) Is the optimized analytical HPLC method good for preparative one? 2) If it is, what is the maximum loading for touching-band separation? Optimization EN 00059 -12 Synergi Hydro-RP (4. 6 × 50 mm) 30 -60% Me. OH/TFA, 5 min, 1. 0 ml/min, 240 nm, 0. 6 mg/ml. Product (MW =471. 5) Anal. Scale-up Synergi Hydro-RP (4. 6 × 100 mm) 30 -60% Me. OH/TFA, 10 min, 1. 0 ml/min, 240 nm, 12. 5 ul, 160 mg/ml. Product This method is not practical for separation of 5 gram samples! (125 prep injections for 17 19 × 100 mm column!)

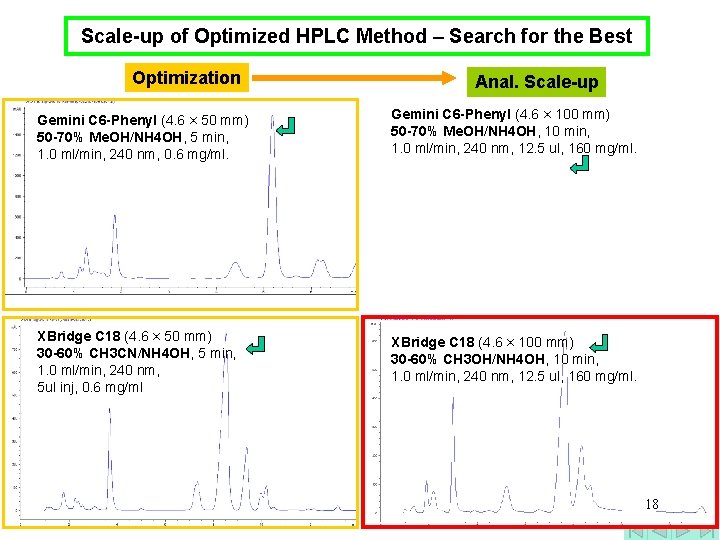
Scale-up of Optimized HPLC Method – Search for the Best Optimization Anal. Scale-up Gemini C 6 -Phenyl (4. 6 × 50 mm) 50 -70% Me. OH/NH 4 OH, 5 min, 1. 0 ml/min, 240 nm, 0. 6 mg/ml. Gemini C 6 -Phenyl (4. 6 × 100 mm) 50 -70% Me. OH/NH 4 OH, 10 min, 1. 0 ml/min, 240 nm, 12. 5 ul, 160 mg/ml. XBridge C 18 (4. 6 × 50 mm) 30 -60% CH 3 CN/NH 4 OH, 5 min, 1. 0 ml/min, 240 nm, 5 ul inj, 0. 6 mg/ml XBridge C 18 (4. 6 × 100 mm) 30 -60% CH 3 OH/NH 4 OH, 10 min, 1. 0 ml/min, 240 nm, 12. 5 ul, 160 mg/ml. 18

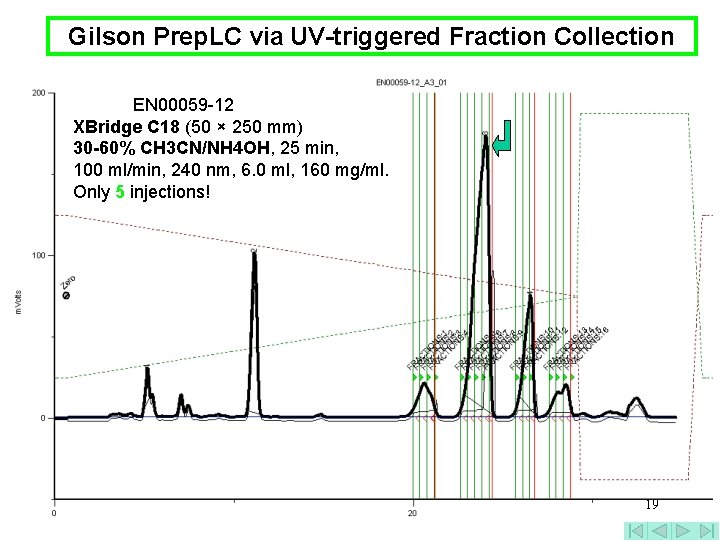
Gilson Prep. LC via UV-triggered Fraction Collection EN 00059 -12 XBridge C 18 (50 × 250 mm) 30 -60% CH 3 CN/NH 4 OH, 25 min, 100 ml/min, 240 nm, 6. 0 ml, 160 mg/ml. Only 5 injections! 19

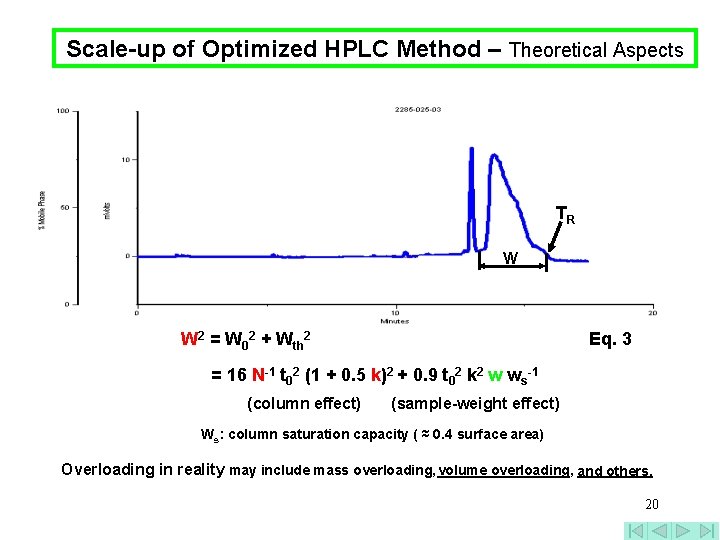
Scale-up of Optimized HPLC Method – Theoretical Aspects TR W W 2 = W 02 + Wth 2 Eq. 3 = 16 N-1 t 02 (1 + 0. 5 k)2 + 0. 9 t 02 k 2 w ws-1 (column effect) (sample-weight effect) Ws: column saturation capacity ( ≈ 0. 4 surface area) Overloading in reality may include mass overloading, volume overloading, and others. 20

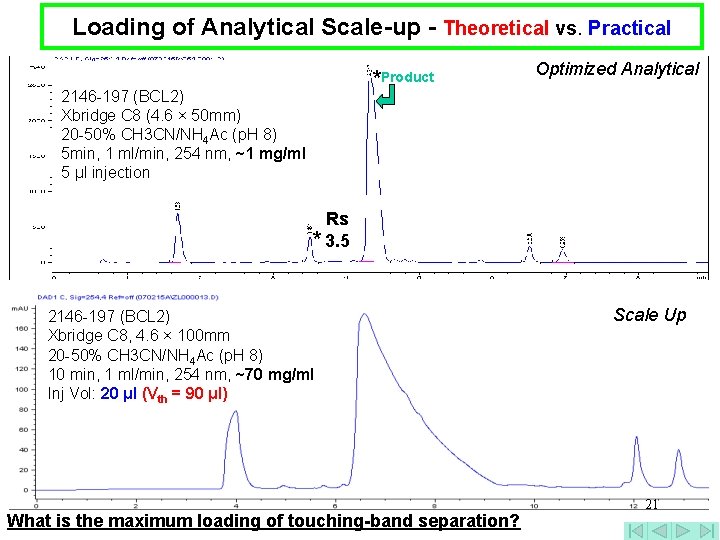
Loading of Analytical Scale-up - Theoretical vs. Practical *Product 2146 -197 (BCL 2) Xbridge C 8 (4. 6 × 50 mm) 20 -50% CH 3 CN/NH 4 Ac (p. H 8) 5 min, 1 ml/min, 254 nm, ~1 mg/ml 5 μl injection Optimized Analytical Rs * 3. 5 2146 -197 (BCL 2) Xbridge C 8, 4. 6 × 100 mm 20 -50% CH 3 CN/NH 4 Ac (p. H 8) 10 min, 1 ml/min, 254 nm, ~70 mg/ml Inj Vol: 20 μl (Vth = 90 μl) What is the maximum loading of touching-band separation? Scale Up 21

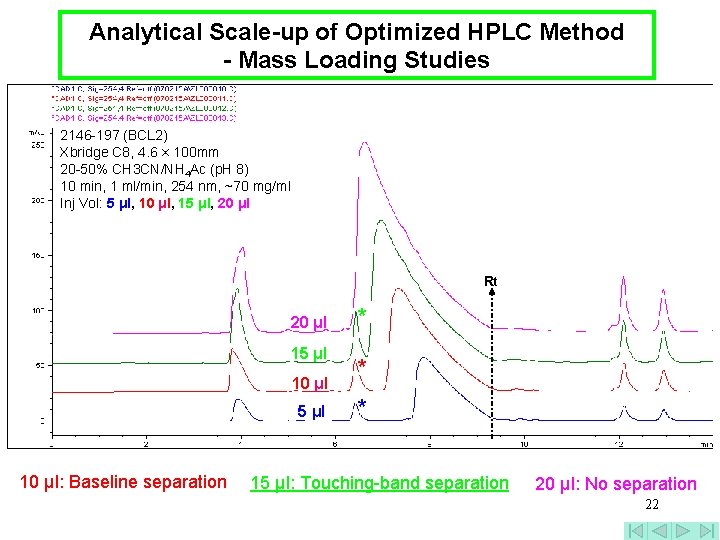
Analytical Scale-up of Optimized HPLC Method - Mass Loading Studies 2146 -197 (BCL 2) Xbridge C 8, 4. 6 × 100 mm 20 -50% CH 3 CN/NH 4 Ac (p. H 8) 10 min, 1 ml/min, 254 nm, ~70 mg/ml Inj Vol: 5 μl, 10 μl, 15 μl, 20 μl Rt 20 μl 15 μl 10 μl: Baseline separation * * * 15 μl: Touching-band separation 20 μl: No separation 22

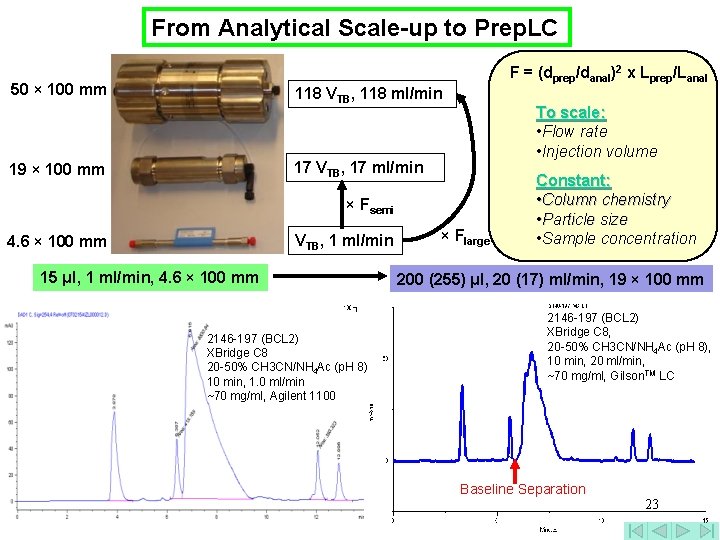
From Analytical Scale-up to Prep. LC F = (dprep/danal)2 x Lprep/Lanal 50 × 100 mm 118 VTB, 118 ml/min To scale: • Flow rate • Injection volume 17 VTB, 17 ml/min 19 × 100 mm × Fsemi VTB, 1 ml/min 4. 6 × 100 mm 15 μl, 1 ml/min, 4. 6 × 100 mm 2146 -197 (BCL 2) XBridge C 8 20 -50% CH 3 CN/NH 4 Ac (p. H 8) 10 min, 1. 0 ml/min ~70 mg/ml, Agilent 1100 × Flarge Constant: • Column chemistry • Particle size • Sample concentration 200 (255) μl, 20 (17) ml/min, 19 × 100 mm 2146 -197 (BCL 2) XBridge C 8, 20 -50% CH 3 CN/NH 4 Ac (p. H 8), 10 min, 20 ml/min, ~70 mg/ml, Gilson. TM LC Baseline Separation 23

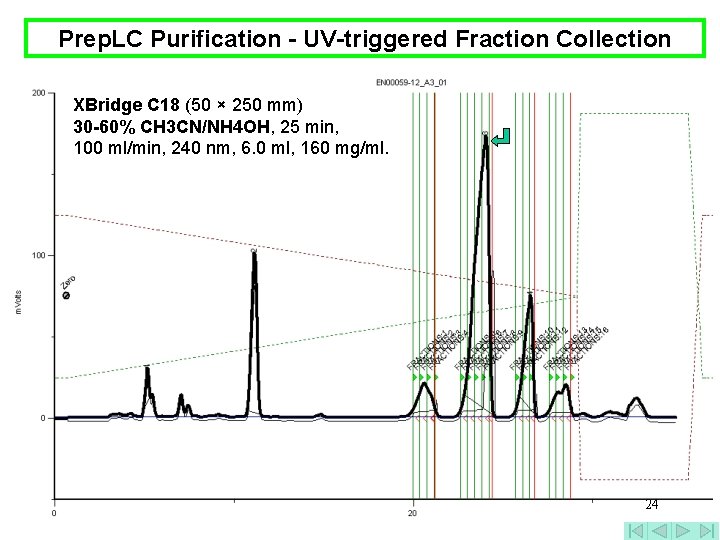
Prep. LC Purification - UV-triggered Fraction Collection XBridge C 18 (50 × 250 mm) 30 -60% CH 3 CN/NH 4 OH, 25 min, 100 ml/min, 240 nm, 6. 0 ml, 160 mg/ml. 24

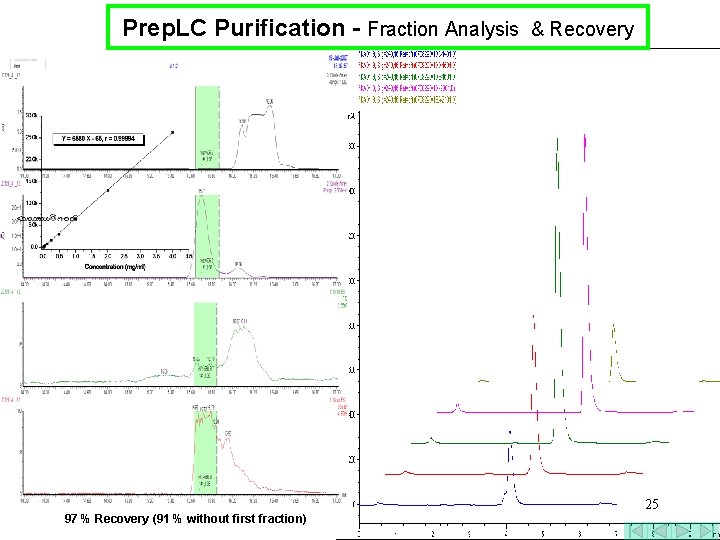
Prep. LC Purification - Fraction Analysis & Recovery 97 % Recovery (91 % without first fraction) 25

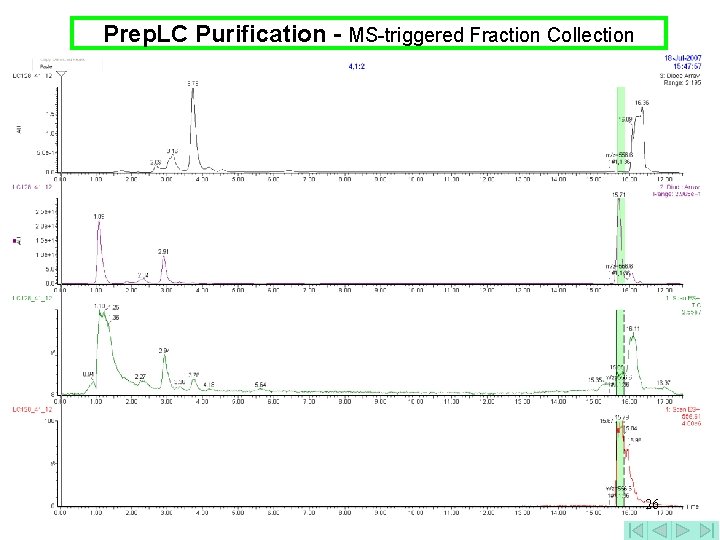
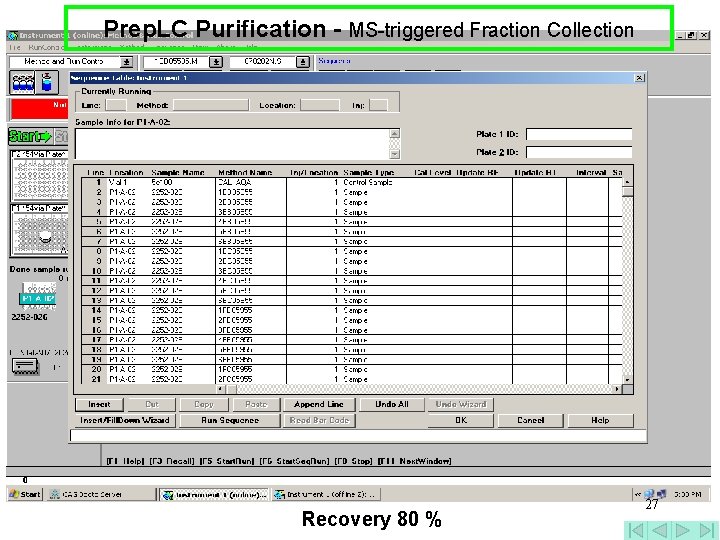
Prep. LC Purification - MS-triggered Fraction Collection 26

Prep. LC Purification - MS-triggered Fraction Collection Recovery 80 % 27

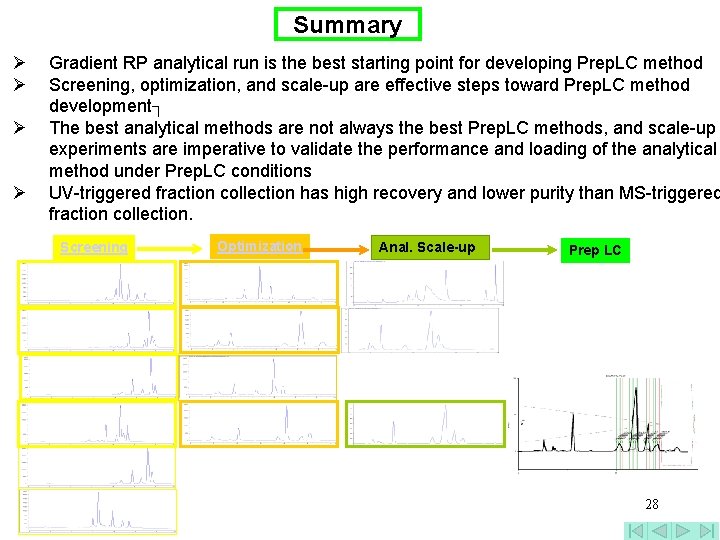
Summary Ø Ø Gradient RP analytical run is the best starting point for developing Prep. LC method Screening, optimization, and scale-up are effective steps toward Prep. LC method development┐ The best analytical methods are not always the best Prep. LC methods, and scale-up experiments are imperative to validate the performance and loading of the analytical method under Prep. LC conditions UV-triggered fraction collection has high recovery and lower purity than MS-triggered fraction collection. Screening Optimization Anal. Scale-up Prep LC 28

Acknowledgment Members of Analytical Group: Tatyana, Camil, Nancy, Mark, Sharon, Milena, Ziling. Randstad USA: Yushen Chang, Vincent Cianciaruso. 29

30

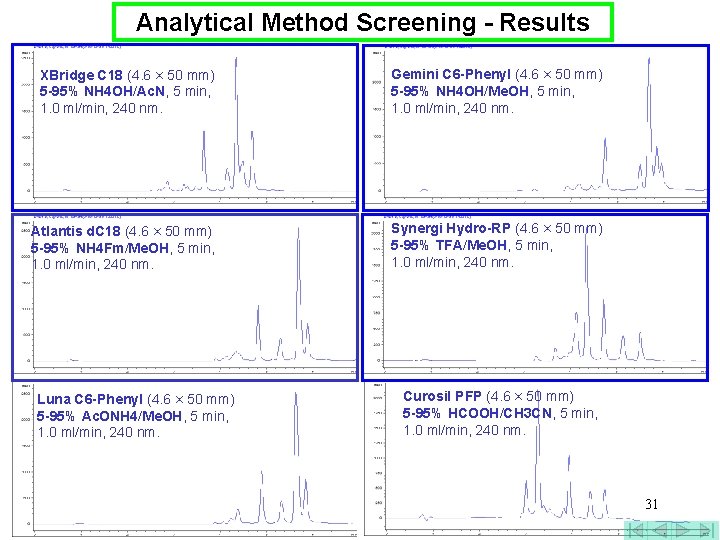
Analytical Method Screening - Results XBridge C 18 (4. 6 × 50 mm) 5 -95% NH 4 OH/Ac. N, 5 min, 1. 0 ml/min, 240 nm. Gemini C 6 -Phenyl (4. 6 × 50 mm) 5 -95% NH 4 OH/Me. OH, 5 min, 1. 0 ml/min, 240 nm. Atlantis d. C 18 (4. 6 × 50 mm) 5 -95% NH 4 Fm/Me. OH, 5 min, 1. 0 ml/min, 240 nm. Synergi Hydro-RP (4. 6 × 50 mm) 5 -95% TFA/Me. OH, 5 min, 1. 0 ml/min, 240 nm. Luna C 6 -Phenyl (4. 6 × 50 mm) 5 -95% Ac. ONH 4/Me. OH, 5 min, 1. 0 ml/min, 240 nm. Curosil PFP (4. 6 × 50 mm) 5 -95% HCOOH/CH 3 CN, 5 min, 1. 0 ml/min, 240 nm. 31

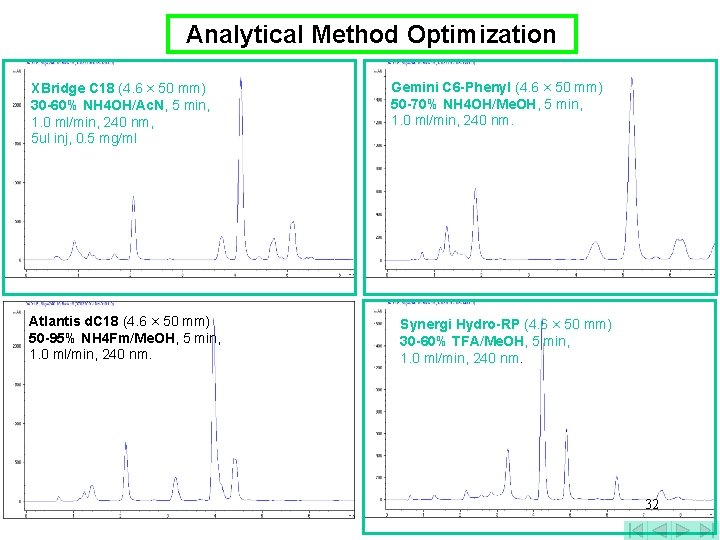
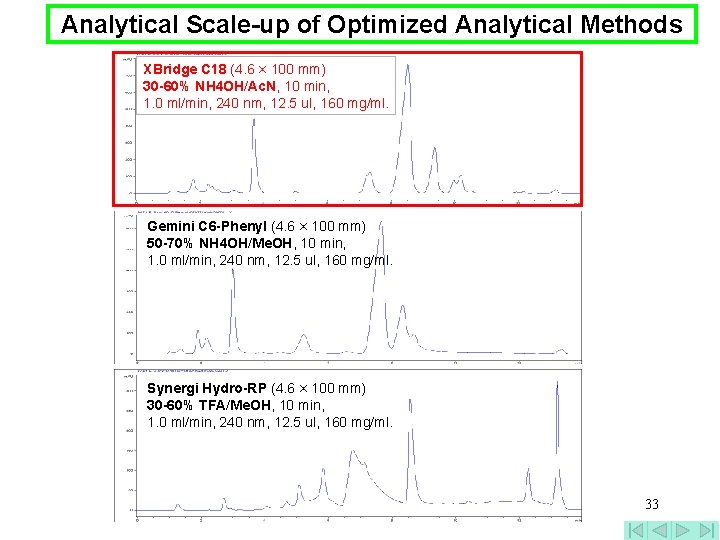
Analytical Method Optimization XBridge C 18 (4. 6 × 50 mm) 30 -60% NH 4 OH/Ac. N, 5 min, 1. 0 ml/min, 240 nm, 5 ul inj, 0. 5 mg/ml Atlantis d. C 18 (4. 6 × 50 mm) 50 -95% NH 4 Fm/Me. OH, 5 min, 1. 0 ml/min, 240 nm. Gemini C 6 -Phenyl (4. 6 × 50 mm) 50 -70% NH 4 OH/Me. OH, 5 min, 1. 0 ml/min, 240 nm. Synergi Hydro-RP (4. 6 × 50 mm) 30 -60% TFA/Me. OH, 5 min, 1. 0 ml/min, 240 nm. 32

Analytical Scale-up of Optimized Analytical Methods XBridge C 18 (4. 6 × 100 mm) 30 -60% NH 4 OH/Ac. N, 10 min, 1. 0 ml/min, 240 nm, 12. 5 ul, 160 mg/ml. Gemini C 6 -Phenyl (4. 6 × 100 mm) 50 -70% NH 4 OH/Me. OH, 10 min, 1. 0 ml/min, 240 nm, 12. 5 ul, 160 mg/ml. Synergi Hydro-RP (4. 6 × 100 mm) 30 -60% TFA/Me. OH, 10 min, 1. 0 ml/min, 240 nm, 12. 5 ul, 160 mg/ml. 33

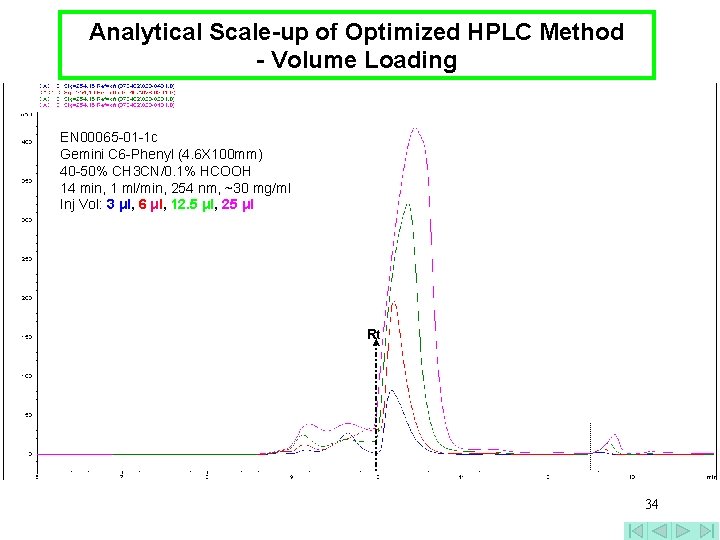
Analytical Scale-up of Optimized HPLC Method - Volume Loading EN 00065 -01 -1 c Gemini C 6 -Phenyl (4. 6 X 100 mm) 40 -50% CH 3 CN/0. 1% HCOOH 14 min, 1 ml/min, 254 nm, ~30 mg/ml Inj Vol: 3 μl, 6 μl, 12. 5 μl, 25 μl Rt 34

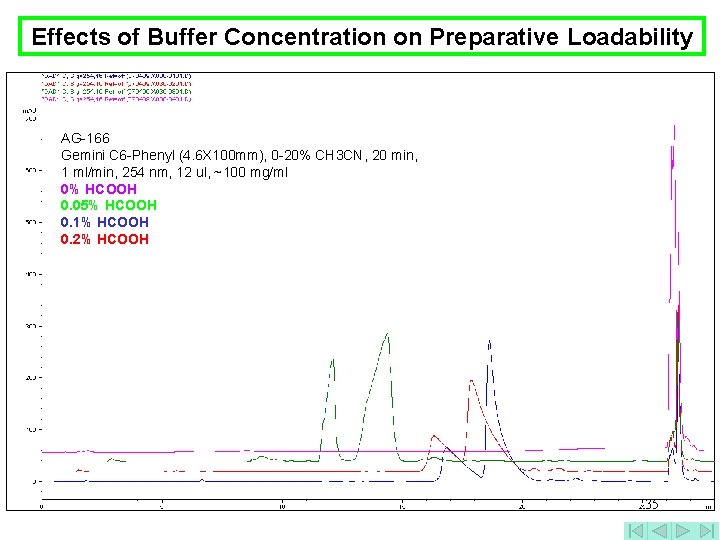
Effects of Buffer Concentration on Preparative Loadability AG-166 Gemini C 6 -Phenyl (4. 6 X 100 mm), 0 -20% CH 3 CN, 20 min, 1 ml/min, 254 nm, 12 ul, ~100 mg/ml 0% HCOOH 0. 05% HCOOH 0. 1% HCOOH 0. 2% HCOOH 35

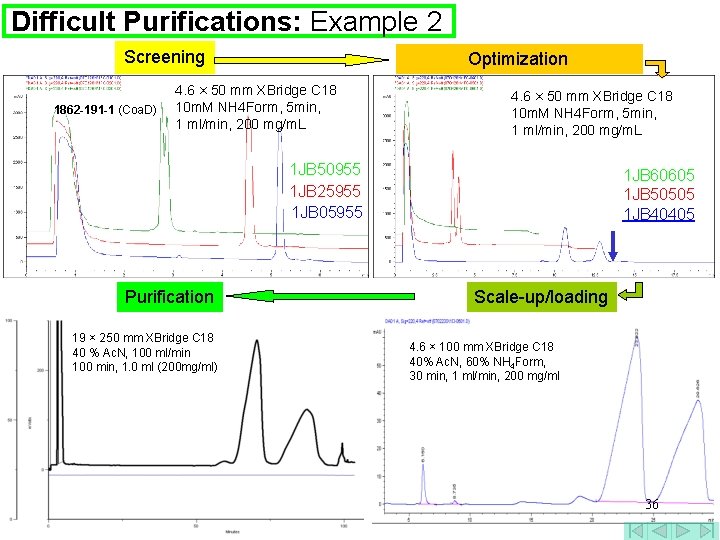
Difficult Purifications: Example 2 Screening 1862 -191 -1 (Coa. D) Optimization 4. 6 × 50 mm XBridge C 18 10 m. M NH 4 Form, 5 min, 1 ml/min, 200 mg/m. L 1 JB 50955 1 JB 25955 1 JB 05955 Purification 19 × 250 mm XBridge C 18 40 % Ac. N, 100 ml/min 100 min, 1. 0 ml (200 mg/ml) 1 JB 60605 1 JB 50505 1 JB 40405 Scale-up/loading 4. 6 × 100 mm XBridge C 18 40% Ac. N, 60% NH 4 Form, 30 min, 1 ml/min, 200 mg/ml 36

Valves: G 1159 A 6 -Col. Selector, G 1160 A, 12/13 Selvalves Detectors: Agilent MSD and Sedex 75 ESDL Detector Finnigan AQA mass spectrometer by closed contact NP Chiral HPLC Solvent: HX, Me. OH/Et. OH(1/1), IPA, 0. 1% Diethylamine Detectors: Advanced Laser Polarimeter, PDR_Chiral Inc Columns: Chiralpak AD Chiralpak OD, Chiralpak AS, Chiralpak IA, Chiralpak IB, Chiralcel OD, Chiralcel OJ, Regis Pirkle covalent (S, S) whelk O 2 10/100 FEC, Regis Pirkle covalent (S, S) whelk O 1 5/100, Regis (S, S) ULMO 5/100, Regis (S, S) DACH DNB 5/100, Phenomenex Chirex ®-PGLY and DNB, Large 5 cm X 50 cm Prep Columns: Chiralpak AD Chiralpak OD, Chiralpak AS, Chiralcel OJ NP Columns: Luna silica 10/100, YMC-PVA-sil 5/120, YMC-Pak Diol 5/60, YMC-Pak CN 5/120, Luna NH 2 5/100, Princeton. SFC Pyridine 5/60 SFC Columns: Berger silica, Diol, CN, Pyridine, Chiralpak AD-H 37

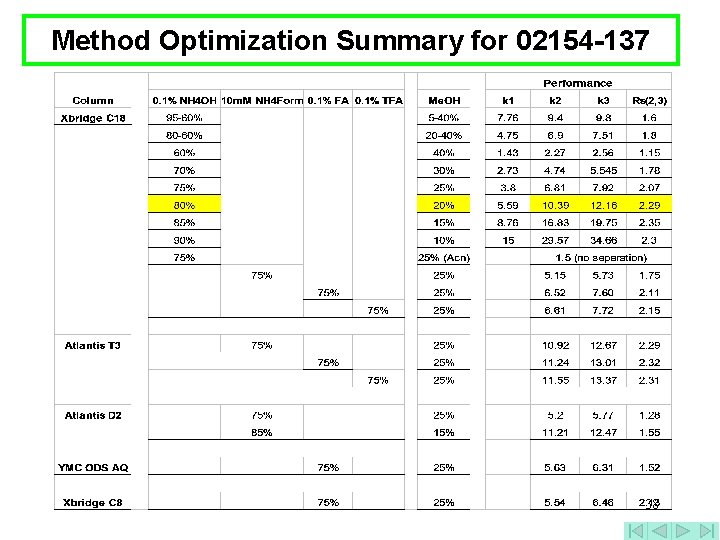
Method Optimization Summary for 02154 -137 38

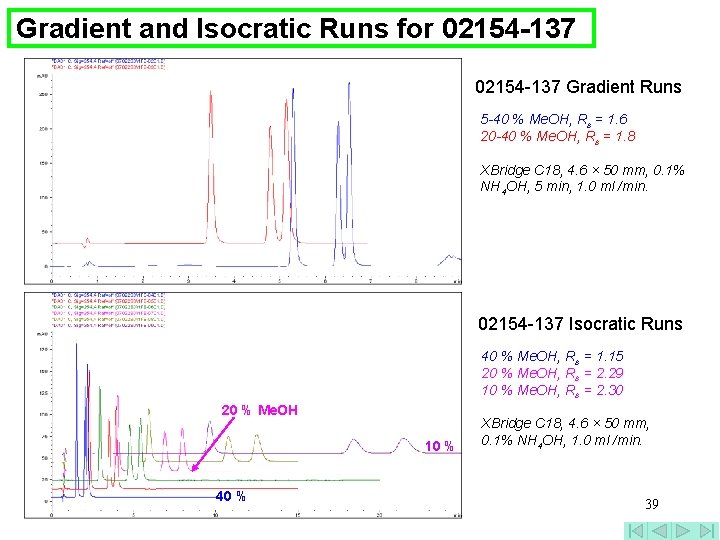
Gradient and Isocratic Runs for 02154 -137 Gradient Runs 5 -40 % Me. OH, Rs = 1. 6 20 -40 % Me. OH, Rs = 1. 8 XBridge C 18, 4. 6 × 50 mm, 0. 1% NH 4 OH, 5 min, 1. 0 ml /min. 02154 -137 Isocratic Runs 40 % Me. OH, Rs = 1. 15 20 % Me. OH, Rs = 2. 29 10 % Me. OH, Rs = 2. 30 20 % Me. OH 10 % 40 % XBridge C 18, 4. 6 × 50 mm, 0. 1% NH 4 OH, 1. 0 ml /min. 39

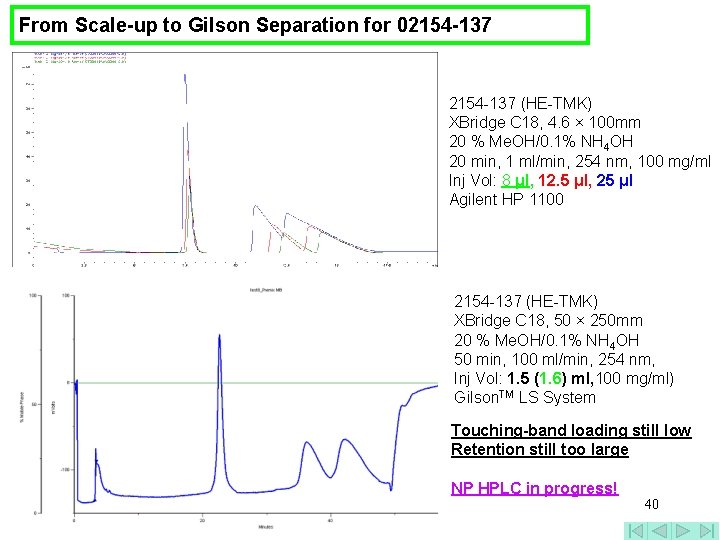
From Scale-up to Gilson Separation for 02154 -137 (HE-TMK) XBridge C 18, 4. 6 × 100 mm 20 % Me. OH/0. 1% NH 4 OH 20 min, 1 ml/min, 254 nm, 100 mg/ml Inj Vol: 8 μl, 12. 5 μl, 25 μl Agilent HP 1100 2154 -137 (HE-TMK) XBridge C 18, 50 × 250 mm 20 % Me. OH/0. 1% NH 4 OH 50 min, 100 ml/min, 254 nm, Inj Vol: 1. 5 (1. 6) ml, 100 mg/ml) Gilson. TM LS System Touching-band loading still low Retention still too large NP HPLC in progress! 40
 Lou cheng
Lou cheng Double pot method water purification
Double pot method water purification Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Edman degradation steps
Edman degradation steps Purification of plasmid
Purification of plasmid Benzaldehyde purification
Benzaldehyde purification Diode is used for purification
Diode is used for purification Inert gas purification
Inert gas purification Purification par recristallisation
Purification par recristallisation Water treatment objectives
Water treatment objectives Introduction of purification of water
Introduction of purification of water Inert gas purification
Inert gas purification Protein purification
Protein purification Cosmid vector slideshare
Cosmid vector slideshare Purification of plasmid
Purification of plasmid Double pot method of water purification
Double pot method of water purification Citrate vs citric acid
Citrate vs citric acid Ion exchange chromatography
Ion exchange chromatography Iises air purification
Iises air purification Purification adn sur colonne de silice
Purification adn sur colonne de silice Edman degradation
Edman degradation Protein purification and characterization techniques
Protein purification and characterization techniques Ngc protein purification
Ngc protein purification List of surfactants and hlb values
List of surfactants and hlb values Feminax ultra and paracetamol
Feminax ultra and paracetamol Purification and characterization of organic compounds
Purification and characterization of organic compounds Continuous purification process
Continuous purification process Plasmid
Plasmid Double pot method of chlorination
Double pot method of chlorination Dna purification overview
Dna purification overview Purification table
Purification table Purification of plasmid
Purification of plasmid Water purification
Water purification Rowpu
Rowpu Need of water purification
Need of water purification Salting out protein purification
Salting out protein purification Lou harvey leeds
Lou harvey leeds Lou tessier
Lou tessier Abc lou note taking
Abc lou note taking Lifeboat 12 by susan hood
Lifeboat 12 by susan hood Lou pai
Lou pai