PROTEIN PURIFICATION INVOLVING A UNIQUE AUTOCLEAVAGE FEATURE OF

















- Slides: 41

PROTEIN PURIFICATION INVOLVING A UNIQUE AUTO-CLEAVAGE FEATURE OF A REPEATED EAAAK PEPTIDE

Interdisciplinary Research of Enzyme Technology Protein Production Structure & Catalytic Mechanism Enzyme Research Bio-conversion Bio-medical application

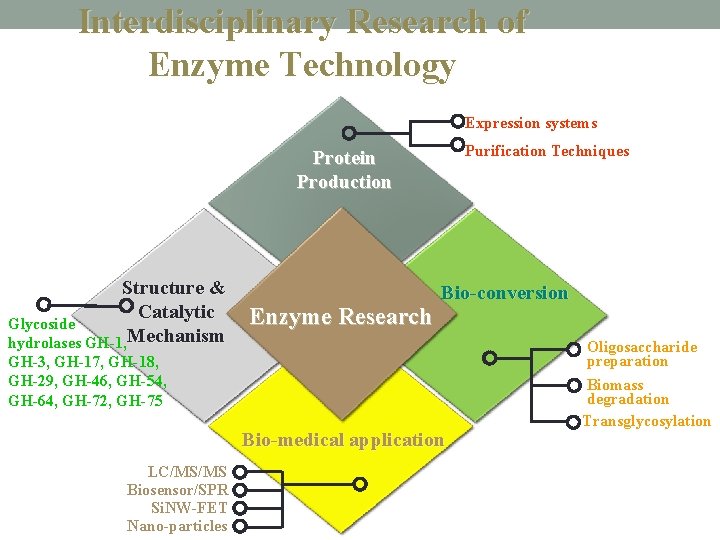
Interdisciplinary Research of Enzyme Technology Expression systems Purification Techniques Protein Production Structure & Catalytic Glycoside hydrolases GH-1, Mechanism Enzyme Research Bio-conversion GH-3, GH-17, GH-18, GH-29, GH-46, GH-54, GH-64, GH-72, GH-75 Bio-medical application LC/MS/MS Biosensor/SPR Si. NW-FET Nano-particles Oligosaccharide preparation Biomass degradation Transglycosylation

PURIFICATION Animal tissue Plant materials Grinding Extraction Fermentation Extracellular enzymes Filtration Concentration Microorganisms Purification Intracellular enzymes Disruption Pure Enzyme

Strategy for massive production and purification of protein

CURRENT STRATEGIES AND PROBLEMS Recombinant protein technology is the best solution so far.

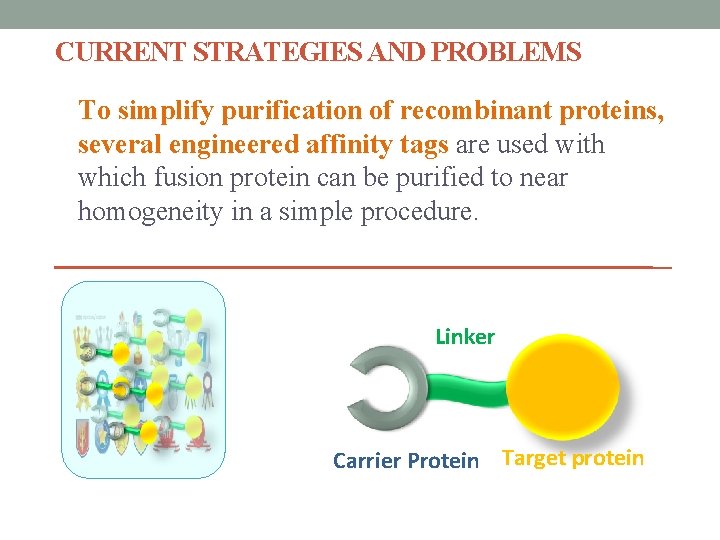
CURRENT STRATEGIES AND PROBLEMS To simplify purification of recombinant proteins, several engineered affinity tags are used with which fusion protein can be purified to near homogeneity in a simple procedure. Linker Carrier Protein Target protein

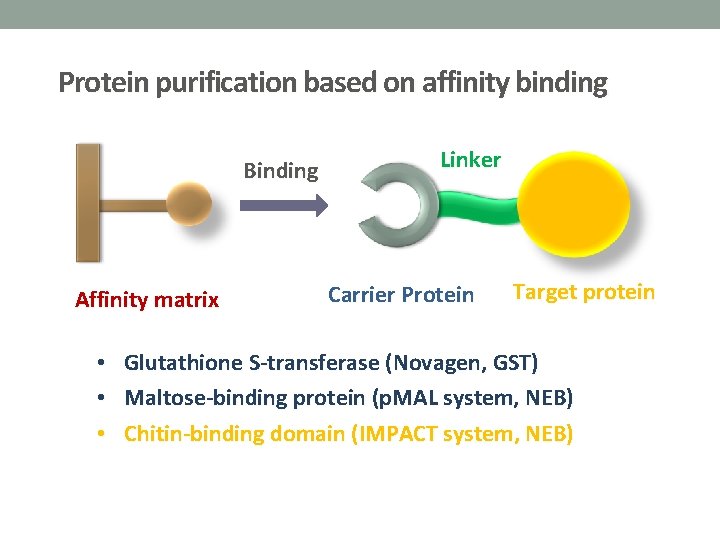
Protein purification based on affinity binding Linker Affinity matrix Carrier Protein Target protein Binding

Protein purification based on affinity binding Binding Affinity matrix Linker Carrier Protein Target protein • Glutathione S-transferase (Novagen, GST) • Maltose-binding protein (p. MAL system, NEB) • Chitin-binding domain (IMPACT system, NEB)

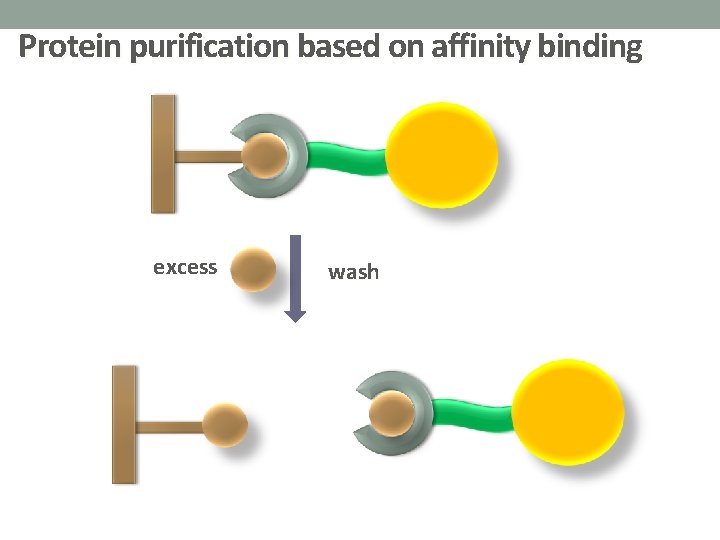
Protein purification based on affinity binding excess wash

Dialysis to remove

CURRENT STRATEGIES Carrier protein (or Tag) may need to be removed, commonly by protease, protease after fusion protein has been purified before subsequent use in downstream application.

Common Drawbacks Linker Affinity matrix • Costly affinity matrix required. • Post proteolytic process needed.

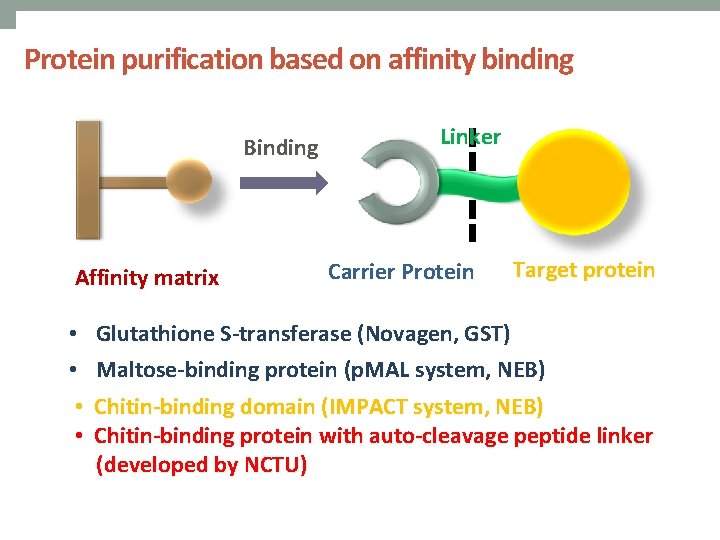
Protein purification based on affinity binding Binding Affinity matrix Linker Carrier Protein Target protein • Glutathione S-transferase (Novagen, GST) • Maltose-binding protein (p. MAL system, NEB) • Chitin-binding domain (IMPACT system, NEB) • Chitin-binding protein with auto-cleavage peptide linker (developed by NCTU)

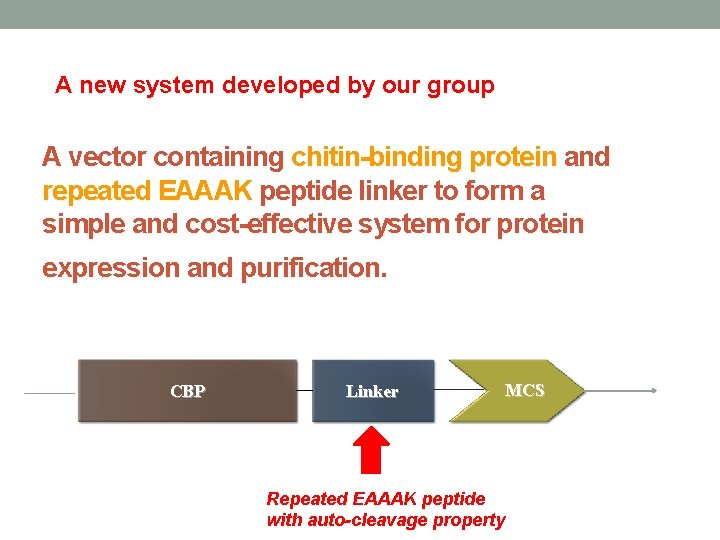
A new system developed by our group A vector containing chitin-binding protein and repeated EAAAK peptide linker to form a simple and cost-effective system for protein expression and purification. CBP Linker MCS Repeated EAAAK peptide with auto-cleavage property

History of our finding…… Starting from the study of Chitinase from Bacillus NCTU 2 Characteristics of chitin-binding Protein (CBP) • CBP promotes the hydrolysis of chitin catalyzed by chitinase. • CBP has good binding specificity for chitin. • p. H> 8, CBP can bind to chitin. • p. H< 6, CBP can be eluted.

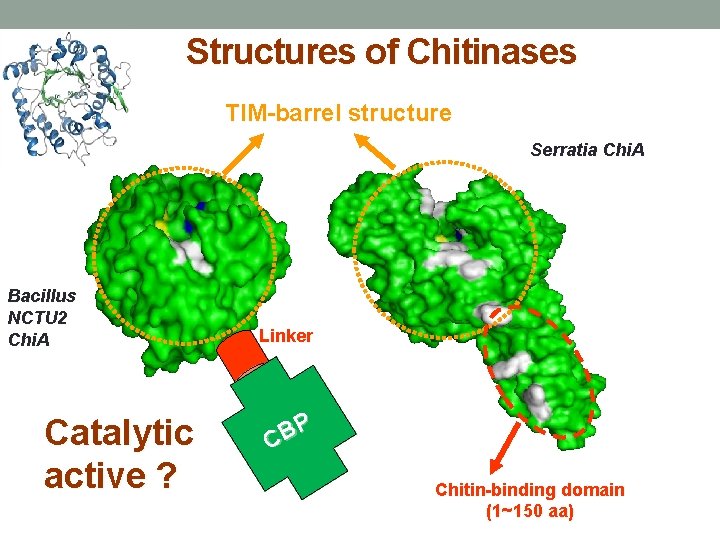
Structures of Chitinases TIM-barrel structure Serratia Chi. A Bacillus NCTU 2 Chi. A Catalytic active ? Linker P B C Chitin-binding domain (1~150 aa)

Vector design

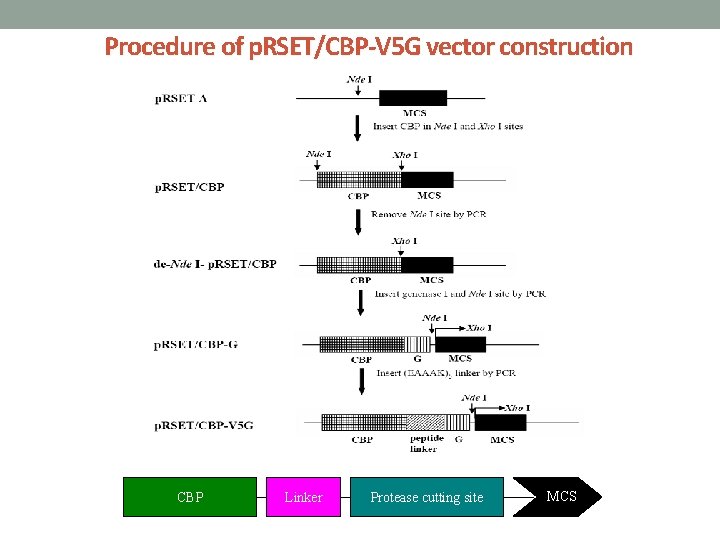
Procedure of p. RSET/CBP-V 5 G vector construction CBP Linker Protease cutting site MCS

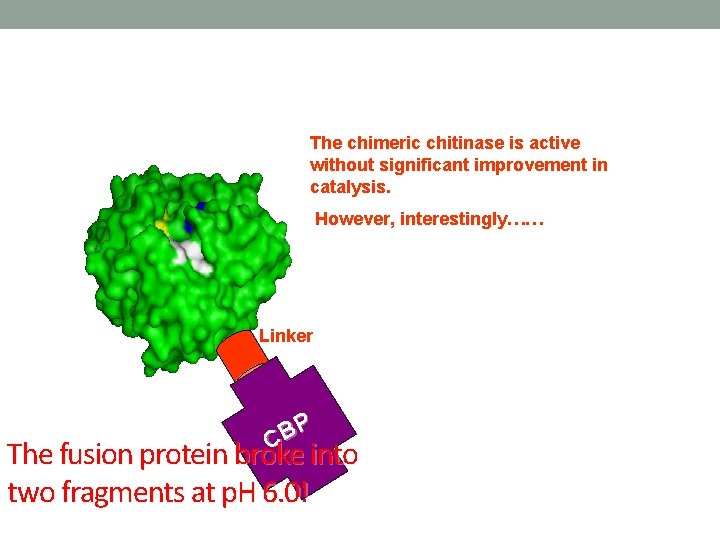
The chimeric chitinase is active without significant improvement in catalysis. However, interestingly…… Linker P B C The fusion protein broke into two fragments at p. H 6. 0!

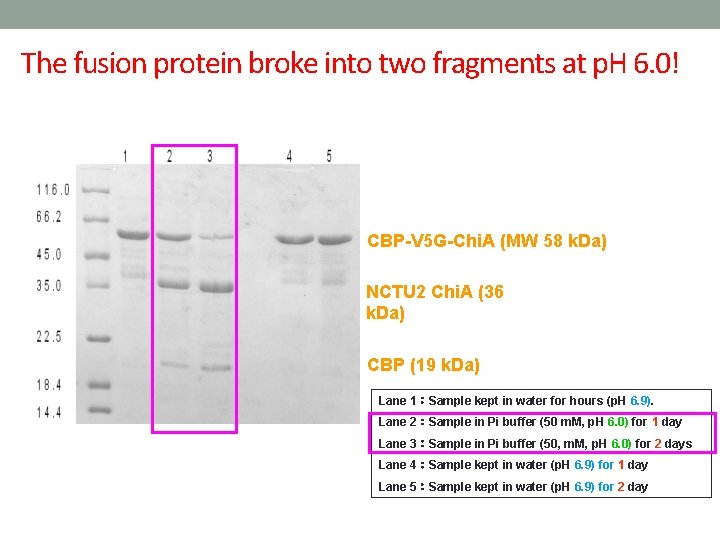
The fusion protein broke into two fragments at p. H 6. 0! CBP-V 5 G-Chi. A (MW 58 k. Da) NCTU 2 Chi. A (36 k. Da) CBP (19 k. Da) Lane 1:Sample kept in water for hours (p. H 6. 9). Lane 2:Sample in Pi buffer (50 m. M, p. H 6. 0) for 1 day Lane 3:Sample in Pi buffer (50, m. M, p. H 6. 0) for 2 days Lane 4:Sample kept in water (p. H 6. 9) for 1 day Lane 5:Sample kept in water (p. H 6. 9) for 2 day

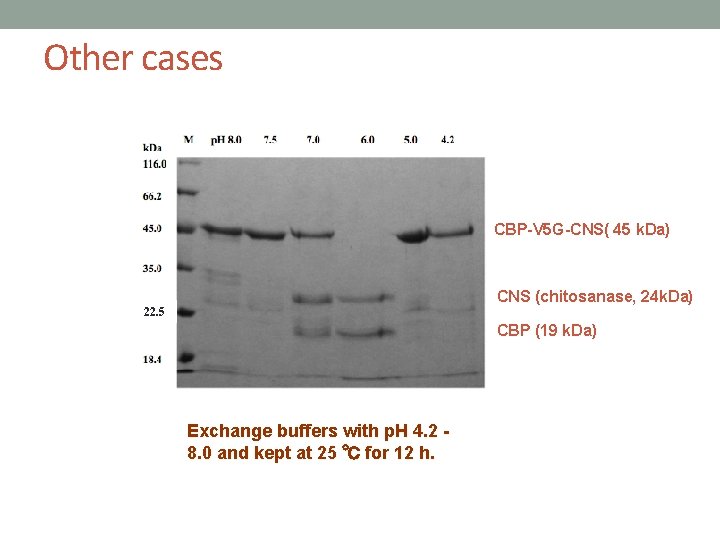
Other cases CBP-V 5 G-CNS( 45 k. Da) CNS (chitosanase, 24 k. Da) 22. 5 CBP (19 k. Da) Exchange buffers with p. H 4. 2 8. 0 and kept at 25 ℃ for 12 h.

Dose Auto-cleavage occur on CBP-(EAAAK)5 -G-Chi. A? Or contamination of protease?

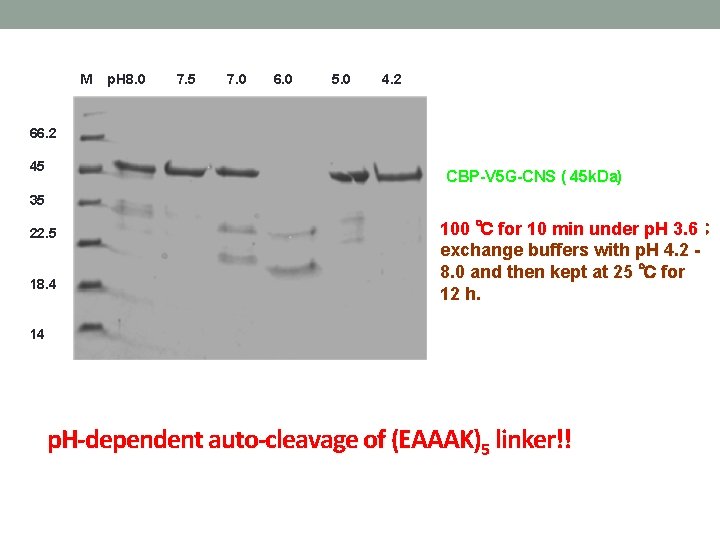
M p. H 8. 0 7. 5 7. 0 6. 0 5. 0 4. 2 66. 2 45 CBP-V 5 G-CNS ( 45 k. Da) 35 22. 5 18. 4 100 ℃ for 10 min under p. H 3. 6; exchange buffers with p. H 4. 2 8. 0 and then kept at 25 ℃ for 12 h. 14 p. H-dependent auto-cleavage of (EAAAK)5 linker!!

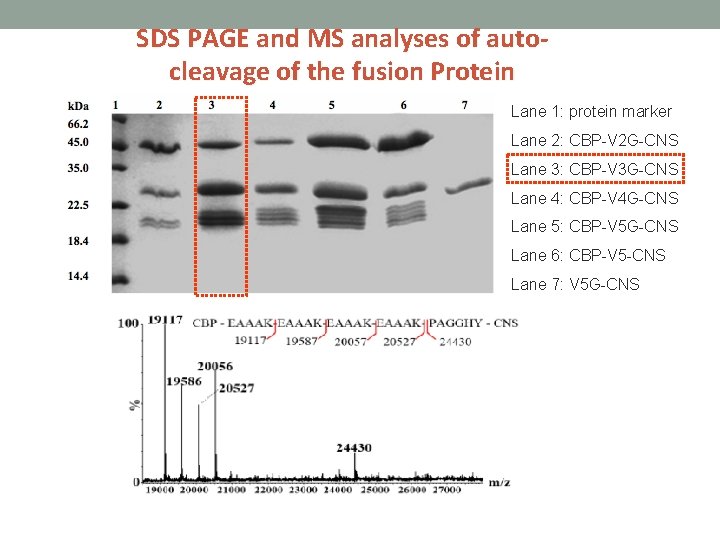
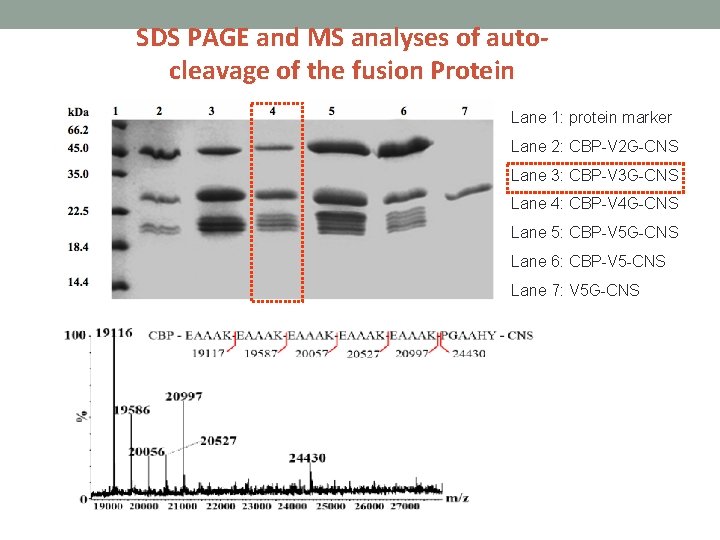
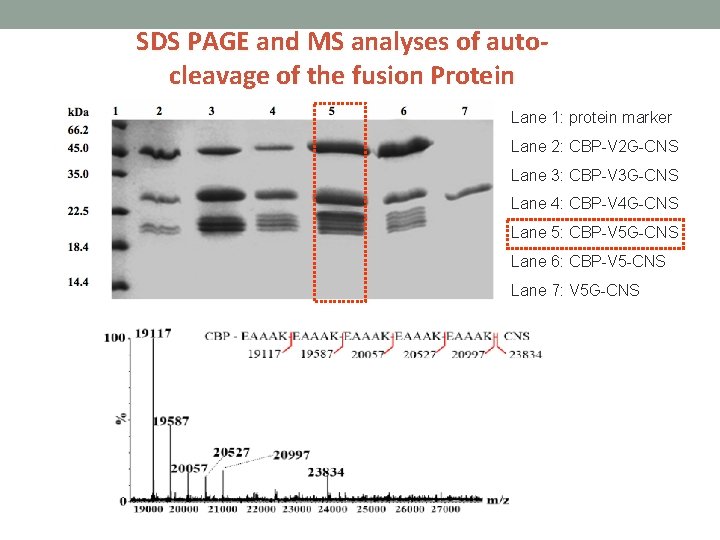
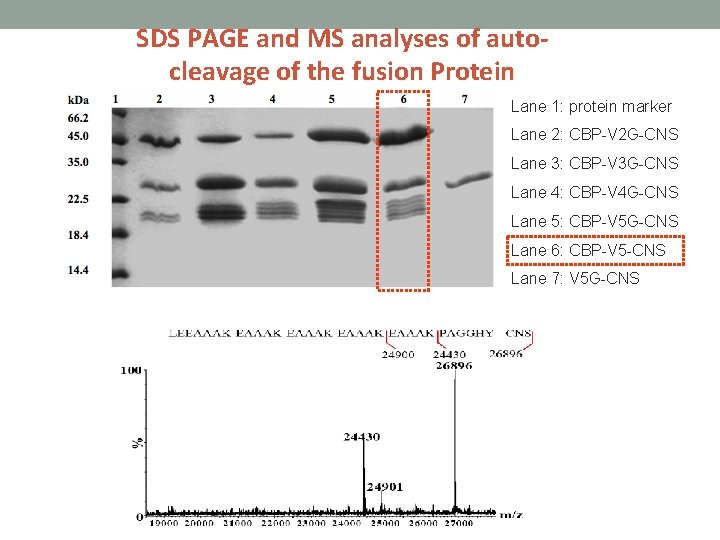
Construction of fusion CNS with various repeated EAAAK linkers • CBP- (EAAAK)2 G-CNS • CBP- (EAAAK)3 G-CNS • CBP- (EAAAK)4 G-CNS • CBP- (EAAAK)5 -CNS (Fusion protein without genenase I cutting site) • (EAAAK)5 G-CNS (Fusion protein without CBP)

The fusion proteins were incubated in phosphate buffer (p. H 6. 0 at 16 ℃) so that partial auto-cleavage fragments can be obtained.

SDS PAGE and MS analyses of auto-cleavage of the fusion Protein Lane 1: protein marker Lane 2: CBP-V 2 G-CNS Lane 3: CBP-V 3 G-CNS Lane 4: CBP-V 4 G-CNS Lane 5: CBP-V 5 G-CNS Lane 6: CBP-V 5 -CNS Lane 7: V 5 G-CNS

SDS PAGE and MS analyses of autocleavage of the fusion Protein Lane 1: protein marker Lane 2: CBP-V 2 G-CNS Lane 3: CBP-V 3 G-CNS Lane 4: CBP-V 4 G-CNS Lane 5: CBP-V 5 G-CNS Lane 6: CBP-V 5 -CNS Lane 7: V 5 G-CNS

SDS PAGE and MS analyses of autocleavage of the fusion Protein Lane 1: protein marker Lane 2: CBP-V 2 G-CNS Lane 3: CBP-V 3 G-CNS Lane 4: CBP-V 4 G-CNS Lane 5: CBP-V 5 G-CNS Lane 6: CBP-V 5 -CNS Lane 7: V 5 G-CNS

SDS PAGE and MS analyses of autocleavage of the fusion Protein Lane 1: protein marker Lane 2: CBP-V 2 G-CNS Lane 3: CBP-V 3 G-CNS Lane 4: CBP-V 4 G-CNS Lane 5: CBP-V 5 G-CNS Lane 6: CBP-V 5 -CNS Lane 7: V 5 G-CNS

SDS PAGE and MS analyses of autocleavage of the fusion Protein Lane 1: protein marker Lane 2: CBP-V 2 G-CNS Lane 3: CBP-V 3 G-CNS Lane 4: CBP-V 4 G-CNS Lane 5: CBP-V 5 G-CNS Lane 6: CBP-V 5 -CNS Lane 7: V 5 G-CNS

SDS PAGE and MS analyses of autocleavage of the fusion Protein Lane 1: protein marker Lane 2: CBP-V 2 G-CNS Lane 3: CBP-V 3 G-CNS Lane 4: CBP-V 4 G-CNS Lane 5: CBP-V 5 G-CNS Lane 6: CBP-V 5 -CNS Lane 7: V 5 G-CNS

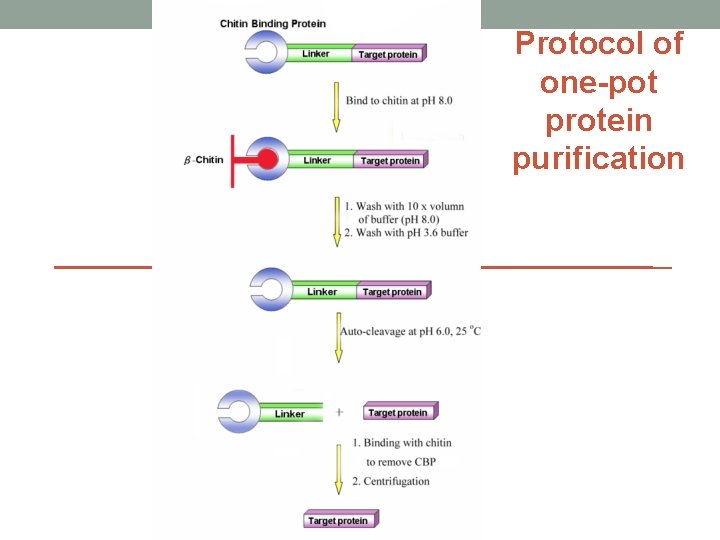
Protocol of one-pot protein purification

CBP-V 5 G-CNS CBP-V 5 G-LPHase Lane 1: marker Lane 2: crude enzyme Lane 3: β-chitin purified enzyme Lane 4: After auto-cleavage, the obtained target protein CNS: 24 k. Da, LPHase: 40 k. Da

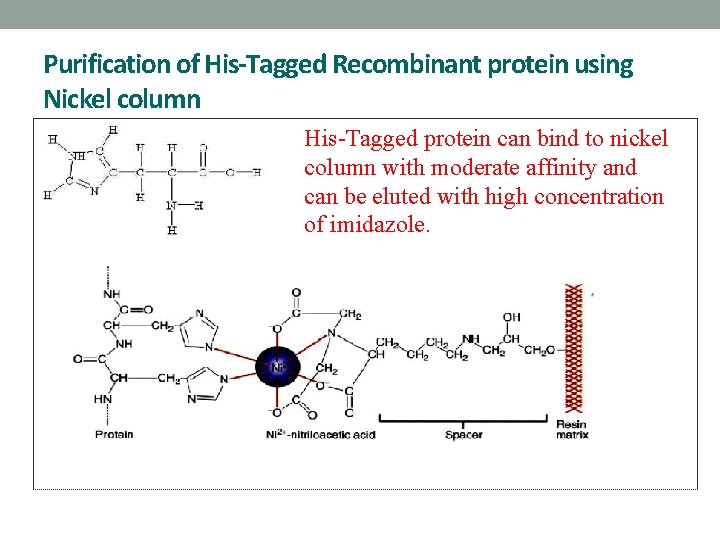
Purification of His-Tagged Recombinant protein using Nickel column His-Tagged protein can bind to nickel column with moderate affinity and can be eluted with high concentration of imidazole.

His-Tag + auto-cleavage peptide + magnetic particles Will it work? ?

One-step protein purification using MP and ACP

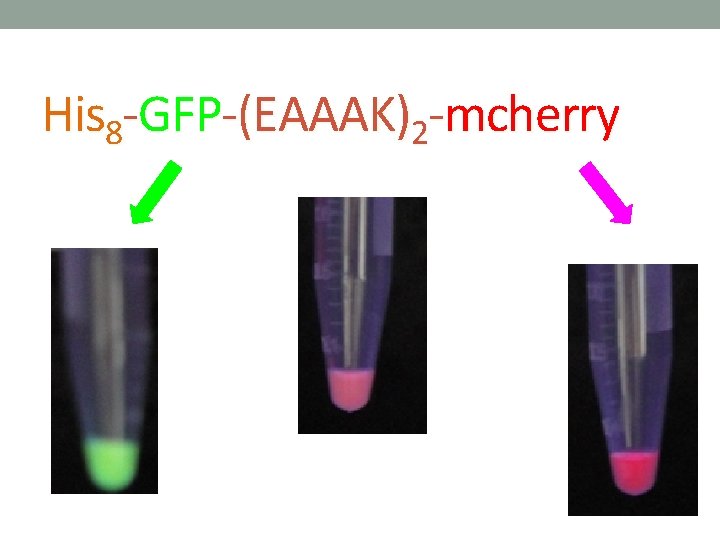
His 8 -GFP-(EAAAK)2 -mcherry

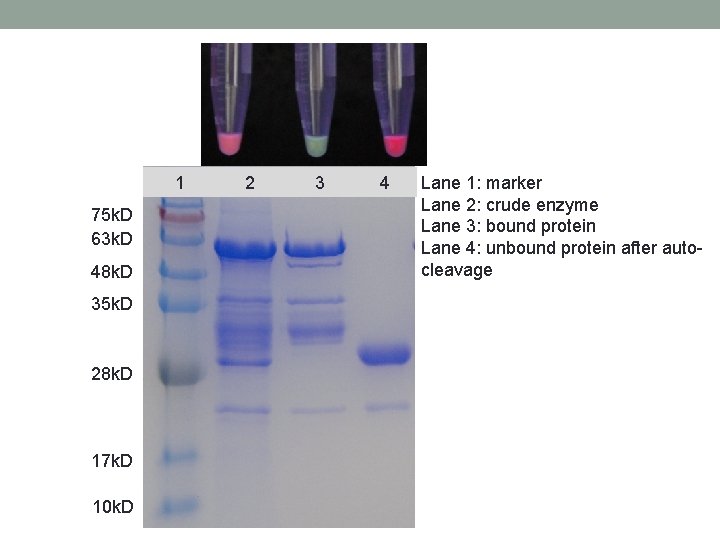
1 75 k. D 63 k. D 48 k. D 35 k. D 28 k. D 17 k. D 10 k. D 2 3 4 Lane 1: marker Lane 2: crude enzyme Lane 3: bound protein Lane 4: unbound protein after autocleavage

His 6 -(EAAAK)3 -GFP 1 2 3 4 100 k. D 75 k. D 63 k. D 48 k. D 35 k. D 28 k. D 17 k. D 10 k. D Lane 1: marker Lane 2: crude enzyme Lane 3: MP bound with protein Lane 4: unbound protein after autocleavage

Conclusions • The repeated EAAAK peptide exhibited an auto-cleavage feature which can be mediated by p. H condition. • With this system, many proteins have been successfully purified. • Integration of auto-cleavage peptide (ACP) technique with NTA -coated magnetic particles coated can simplify the purification process.
 Enzyme purification
Enzyme purification Protein purification and characterization techniques
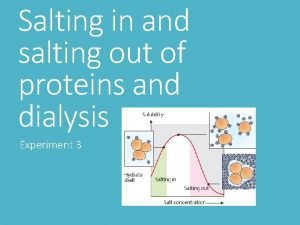
Protein purification and characterization techniques Salting in and salting out of proteins
Salting in and salting out of proteins Ngc protein purification
Ngc protein purification Features of planet mercury
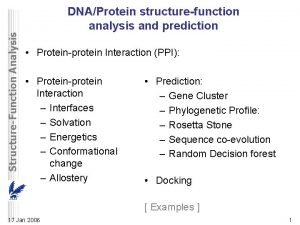
Features of planet mercury Protein-protein docking
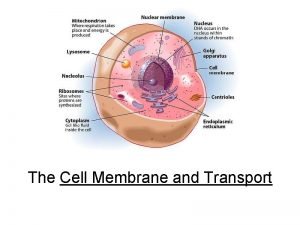
Protein-protein docking Carrier vs channel proteins
Carrier vs channel proteins Feature dataset vs feature class
Feature dataset vs feature class Isolated feature combined feature effects
Isolated feature combined feature effects Diode is used for purification
Diode is used for purification Double pot method of water purification
Double pot method of water purification Inert gas purification
Inert gas purification Gfp purification flow chart
Gfp purification flow chart What are the objectives of water purification
What are the objectives of water purification Purification of plasmid
Purification of plasmid Inert gas purification
Inert gas purification Orthotolidine arsenite test
Orthotolidine arsenite test Cosmids
Cosmids 200 m.g.a.d full form
200 m.g.a.d full form Purification of benzaldehyde
Purification of benzaldehyde Citric acid discovery
Citric acid discovery Continuous purification process
Continuous purification process Iises air purification
Iises air purification What are plasmid
What are plasmid Salting out proteins
Salting out proteins Introduction of purification of water
Introduction of purification of water Purification of plasmid
Purification of plasmid Feminax ultra superdrug
Feminax ultra superdrug Purification and characterization of organic compounds
Purification and characterization of organic compounds Plasmid
Plasmid Glycerol purification
Glycerol purification Dna purification overview
Dna purification overview Purification adn sur colonne de silice
Purification adn sur colonne de silice Gel filtration
Gel filtration Purification par recristallisation
Purification par recristallisation Rowpu 1500 technical order
Rowpu 1500 technical order Edman degradation steps
Edman degradation steps Need of water purification
Need of water purification Water purification
Water purification Three balls of equal mass start from rest
Three balls of equal mass start from rest Spring potential and kinetic energy
Spring potential and kinetic energy Hklp
Hklp