DNA EXTRACTION Protocol and notes 11242020 1 Introduction








- Slides: 11

DNA EXTRACTION Protocol and notes 11/24/2020 1

Introduction • This highlights a simple protocol for extracting DNA from cereal plant leaves • This protocol will produce a product that is suitable as a template for polymerase chain reaction (PCR) and restriction enzyme digestion • It will also contain RNA which can be removed with further purifications 11/24/2020 2

DNA Extraction Protocol Overview • Fresh plant material is collected • The plant material is mixed with extraction buffer and ground in a mortar and pestle • The resulting liquid is centrifuged to separate the solid plant material from the extraction buffer supernatant • The supernatant is mixed with ethanol to precipitate the DNA which will form a pellet at the bottom of the tube • The supernatant is discarded • The DNA pellet is washed with a diluted ethanol solution • The DNA pellet is dried and then dissolved in water or a buffer 11/24/2020 3

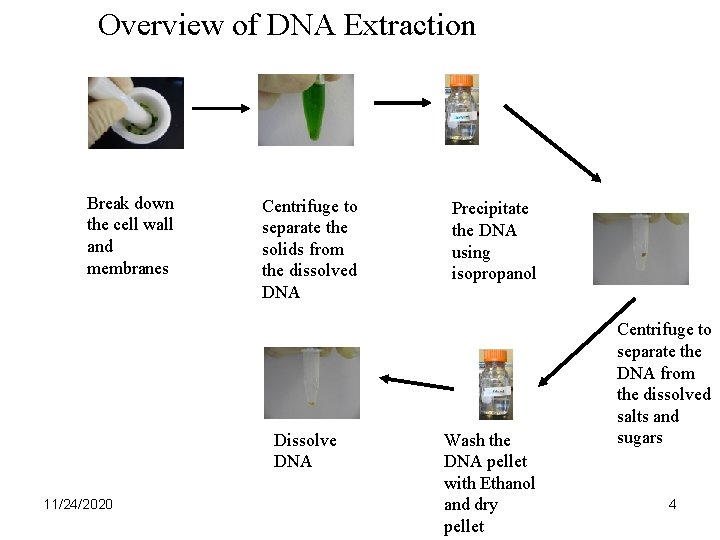
Overview of DNA Extraction Break down the cell wall and membranes Centrifuge to separate the solids from the dissolved DNA Dissolve DNA 11/24/2020 Precipitate the DNA using isopropanol Wash the DNA pellet with Ethanol and dry pellet Centrifuge to separate the DNA from the dissolved salts and sugars 4

Protocol for Collecting the Plant Material 1. Set up and label (according to species) TWO eppendorf tubes for each seedling you will be extracting DNA from. 2. Add 600 µL of isopropanol to one tube in step 1 and leave the other tube empty. 3. Add 2. 0 m. L of extraction buffer to the mortar. 4. Cut a 2 inch portion of leaf from each plant and grind in the mortar with the pestle until the solution is deep green. 11/24/2020 Transfer 1. 5 m. L of solution to empty tube. 5

Protocol (continued) 5. Centrifuge the eppendorf tube with the extraction buffer + crushed leaf at high speed for 3 minutes (this will pellet the leaf debris at the bottom of the tube) 6. Remove 1 m. L of the supernatant and transfer to the tube containing 600 µL of isopropanol. DISCARD the tube with the debris pellet! A centrifuge 11/24/2020 Product is the supernatant 6

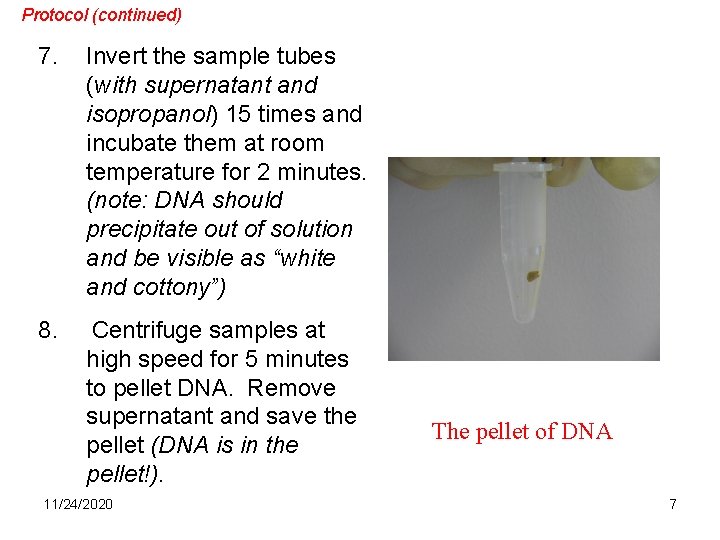
Protocol (continued) 7. Invert the sample tubes (with supernatant and isopropanol) 15 times and incubate them at room temperature for 2 minutes. (note: DNA should precipitate out of solution and be visible as “white and cottony”) 8. Centrifuge samples at high speed for 5 minutes to pellet DNA. Remove supernatant and save the pellet (DNA is in the pellet!). 11/24/2020 The pellet of DNA 7

Protocol (continued) 9. Add 1000 µL of 70% ethanol to the DNA pellets. Mix by inversion 15 times. Centrifuge at high speed for 3 minutes. Remove and discard as much supernatant as possible. Again, save the pellet! 10. Leave the tube open and let it air dry for 10 -15 minutes. Add 50 µL of 1 X TE to resuspend the DNA in solution. Pipette tip being used to break up the DNA pellet Some tips on resuspending the DNA pellet: • Use a pipette tip to break up the pellet to ease resuspension • Do not pipette DNA up & down repeatedly- this shears DNA • It is best to leave the DNA on ice overnight and let it slowly redissolve 11/24/2020 8

Practical Notes • The DNA solution is now ready to use. • To keep DNA solution stable, keep it in the fridge for short term storage and in the freezer for long term storage. • After extractions, it is a good practice to run the DNA on an agarose gel to check its integrity and quality • Mix 10 µL of DNA with 10 µL of loading buffer • Load this mixture into a 1% agarose gel 11/24/2020 9

Examining the Quality of the DNA Extracted The product of your DNA extraction will be used in subsequent experiments, poor quality DNA will not perform well in PCR or restrictive digests. You need to be able to assess the quality of your DNA extraction. Note that genomic DNA has a very high bp, so one would expect a band at the top of the gel (near the well) if it was extracted correctly. 11/24/2020 Ladder A B C D E Barley(A), Corn (B), Oat (C), and Rice (D) are all suitable Wheat (E) may need to be re-extracted 10

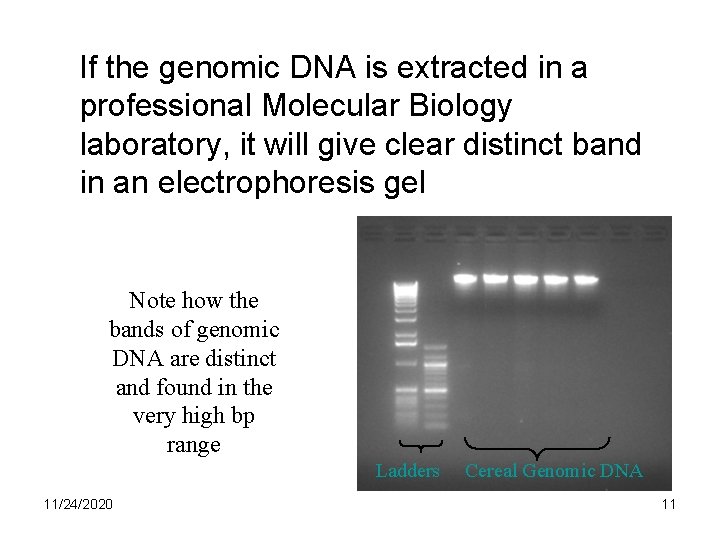
If the genomic DNA is extracted in a professional Molecular Biology laboratory, it will give clear distinct band in an electrophoresis gel Note how the bands of genomic DNA are distinct and found in the very high bp range Ladders 11/24/2020 Cereal Genomic DNA 11