Reactions in Aqueous Solutions Chapter 4 GENERAL PROPERTIES




























- Slides: 43

Reactions in Aqueous Solutions Chapter 4

GENERAL PROPERTIES

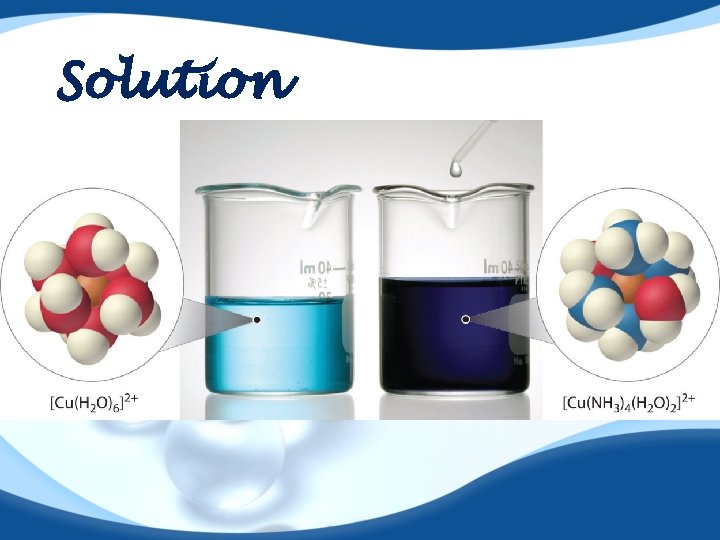
Solution

Electrolyte

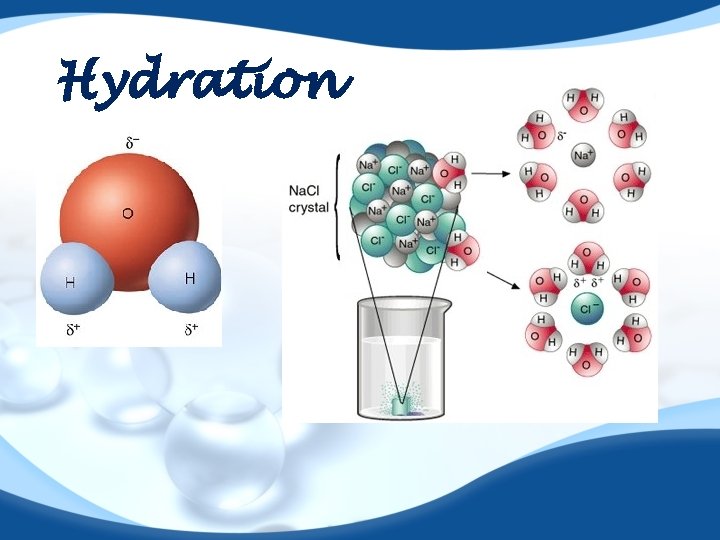
Hydration

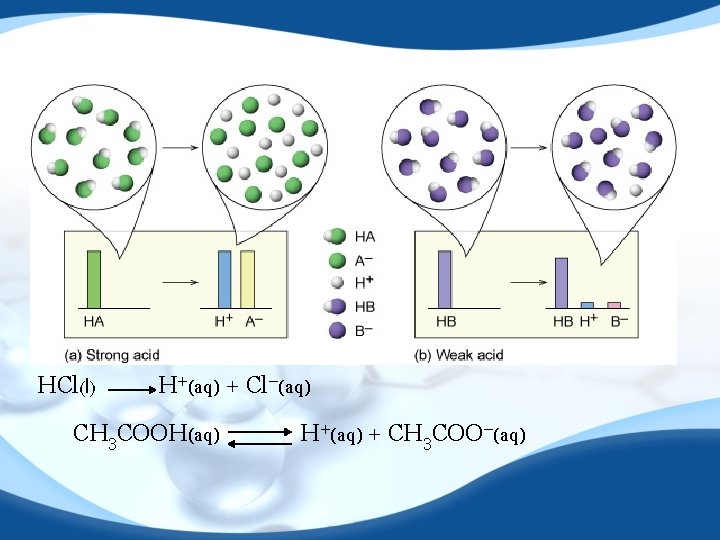
HCl(l) H+(aq) + Cl−(aq) CH 3 COOH(aq) H+(aq) + CH 3 COO−(aq)

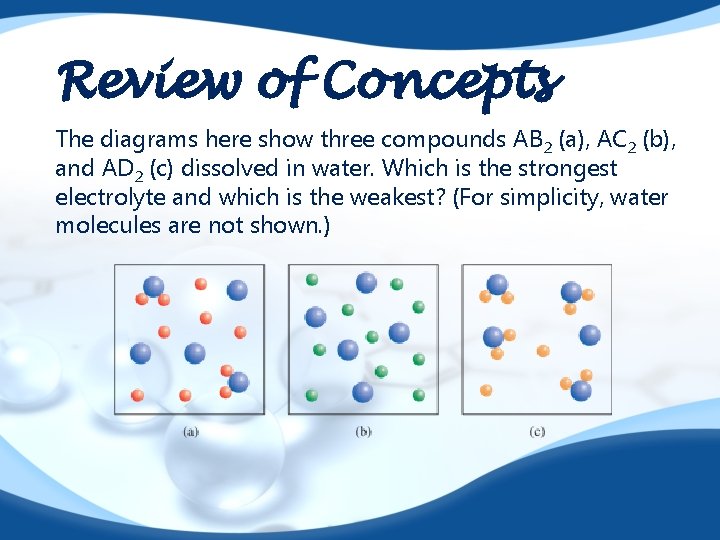
Review of Concepts The diagrams here show three compounds AB 2 (a), AC 2 (b), and AD 2 (c) dissolved in water. Which is the strongest electrolyte and which is the weakest? (For simplicity, water molecules are not shown. )

PRECIPITATE REACTIONS

Double-displacement reaction

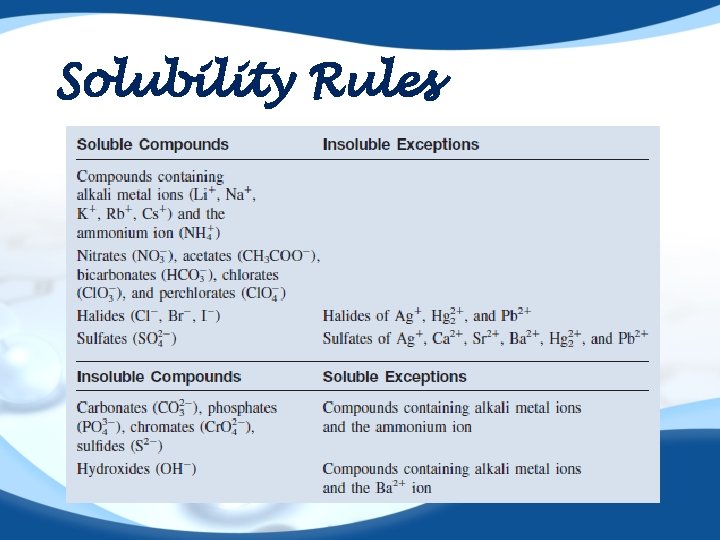
Solubility Rules

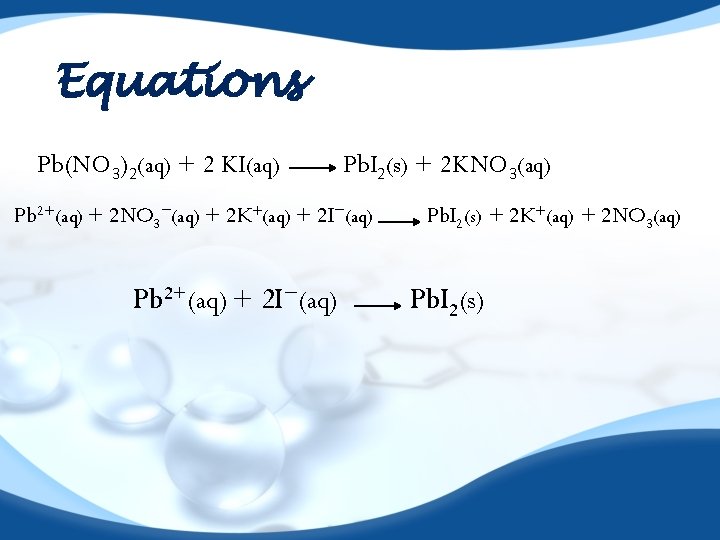
Equations Pb(NO 3)2(aq) + 2 KI(aq) Pb. I 2(s) + 2 KNO 3(aq) Pb 2+(aq) + 2 NO 3−(aq) + 2 K+(aq) + 2 I−(aq) Pb 2+(aq) + 2 I−(aq) Pb. I 2(s) + 2 K+(aq) + 2 NO 3(aq) Pb. I 2(s)

Example K 3 PO 4(aq) + Ca(NO 3)2(aq) Example 4. 2 page 125 in textbook

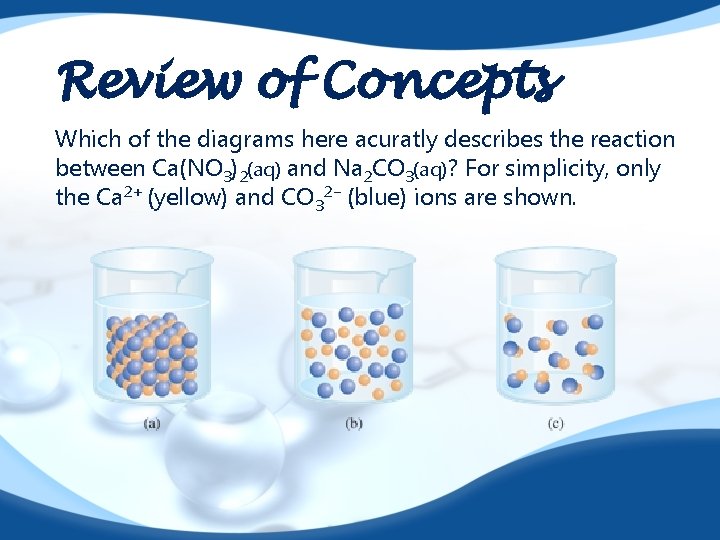
Review of Concepts Which of the diagrams here acuratly describes the reaction between Ca(NO 3)2(aq) and Na 2 CO 3(aq)? For simplicity, only the Ca 2+ (yellow) and CO 32− (blue) ions are shown.

ACID-BASE REACTIONS

General Properties ACID • • • Sour taste Color changes in plant dyes React with metals to produce H 2 gas React with carbonates and bicarbonates to produce CO 2 gas Aqueous acid solutions conduct electricity BASE • • Taste bitter Feel slippery Color changes in plant dyes Aqueous base solutions conduct electricity

Brønsted Acid and Bases ACID • Proton donor • Monoprotic • Diprotic • Triprotic BASE • Proton acceptor

STRONG ACIDS STRONG BASES HI HBr HCl. O 4 HCl H 2 SO 4 HNO 3 Na. OH KOH Li. OH Rb. OH Cs. OH Ca(OH)2 Ba(OH)2 Sr(OH)2 Strong acids/bases are strong electrolytes and will completely dissociate in water.

Review of Concepts Which of the following diagrams best represents a weak acid? Very weak acid? Strong acid? The proton exists in water as the hydronium ion. All acids are monoprotic. (For simplicity, water molecules are not shown. )

Acid-Base Neutralization Reaction between an acid and a base • Generally aqueous solutions result in water and a salt • Ex: HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) *this is a strong acid and strong base so they completely dissociate and the net ionic equation is H+(aq) + OH−(aq) H 2 O(l) • Ex: HCN(aq) + Na. OH(aq) Na. CN(aq) + H 2 O(l) *this is a weak acid and strong base so the acid does not completely ionize in water. When writing the ionic and net ionic equations you cannot break the weak acid apart! The net ionic equation is HCN(aq) + OH−(aq) CN−(aq) + H 2 O(l) •

Gas formation • Certain salts react with acids to produce gaseous products • HNO 3 breaks down into H 2 O(l) + NO 2(g) + NO(g) • H 2 CO 3 breaks down into H 2 O(l) + CO 2(g) • H 2 SO 3 breaks down into H 2 O(l) + SO 2(g) • NH 4 OH breaks down into H 2 O(l) + NH 3(g) • H 2 S(g) • CO 2(g) • H 2(g) • If you get one of these as a product in your molecular equation, they immediately breakdown as above • Gasses do not ionize

Double Replacement Rxns Review Driving Force How do you recognize it? Precipitate You must memorize the solubility rules. Any compound formed from two ions can be recognized as soluble (written as separate ions) or as a precipitate (written as a molecule). Gas formed You must memorize the combinations that decompose into gases (there are 4). You must also memorize the gases that form. For example, when you H 2 SO 3 as a product, you must know it decomposes into H 2 O and SO 2 gas. Weak electrolyte You must memorize the short list of strong acids and strong bases so you will recognize all the weak acids and bases that dissolve, but do not dissociate into ions. The weak base ammonia, NH 3, is in this category. It exits in water as NH 3(aq) and only slightly forms the ions NH 4+ + OH−

OXIDATION-REDUCTION REACTIONS

OIL RIG Half-reaction OXIDATION REACTION • • Reaction that involves the loss of electrons Contains reducing agentdonates electrons REDUCTION REACTION • • Involves the gain of electrons Contains oxidizing agentaccepts electrons

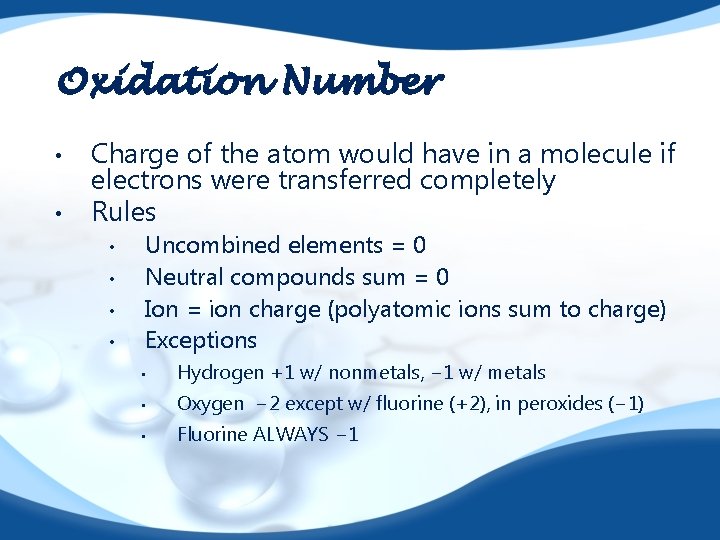
Oxidation Number • • Charge of the atom would have in a molecule if electrons were transferred completely Rules • • Uncombined elements = 0 Neutral compounds sum = 0 Ion = ion charge (polyatomic ions sum to charge) Exceptions • Hydrogen +1 w/ nonmetals, − 1 w/ metals • Oxygen − 2 except w/ fluorine (+2), in peroxides (− 1) • Fluorine ALWAYS − 1

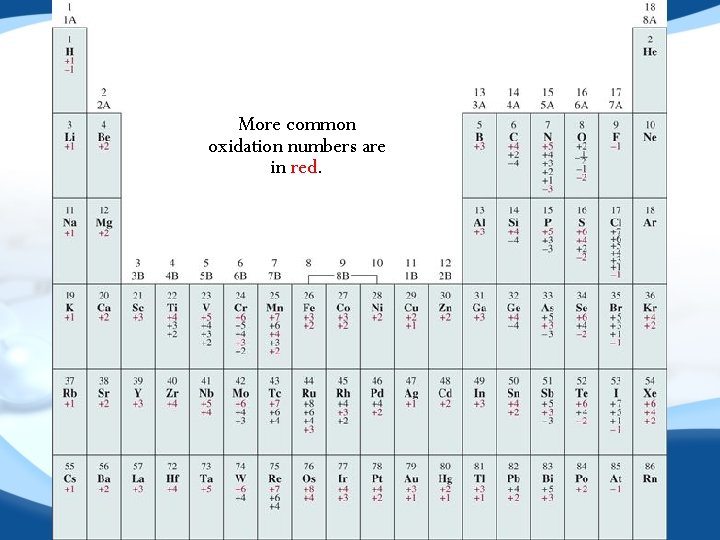
More common oxidation numbers are in red.

Example 2 Mg(s) + O 2(g) 2 Mg. O(s)

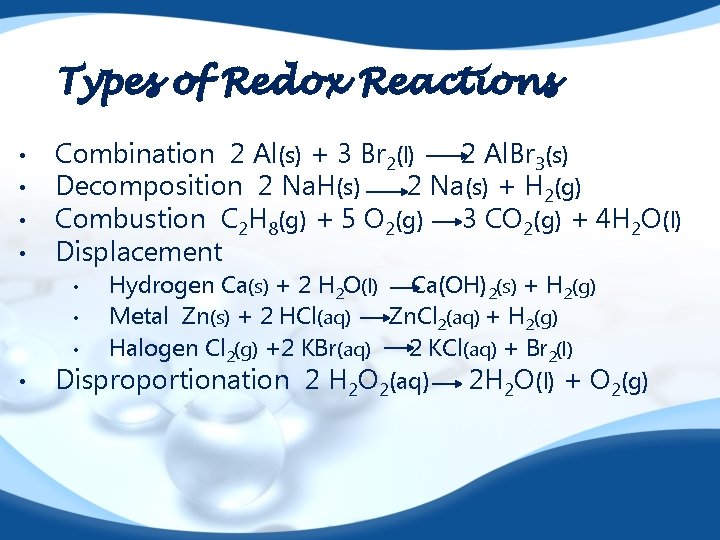
Types of Redox Reactions • • Combination 2 Al(s) + 3 Br 2(l) 2 Al. Br 3(s) Decomposition 2 Na. H(s) 2 Na(s) + H 2(g) Combustion C 2 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(l) Displacement Hydrogen Ca(s) + 2 H 2 O(l) Ca(OH)2(s) + H 2(g) • Metal Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) • Halogen Cl 2(g) +2 KBr(aq) 2 KCl(aq) + Br 2(l) Disproportionation 2 H 2 O 2(aq) 2 H 2 O(l) + O 2(g) • •

Activity Series For Halogens: F 2 > Cl 2 > Br 2 > I 2

Elements most likely to undergo disproportionation

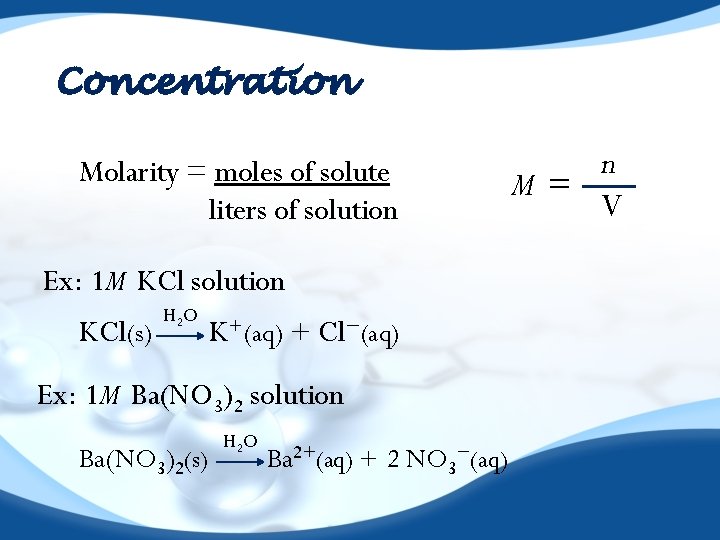
Concentration Molarity = moles of solute liters of solution Ex: 1 M KCl solution KCl(s) H 2 O K+(aq) + Cl−(aq) Ex: 1 M Ba(NO 3)2 solution Ba(NO 3)2(s) H 2 O Ba 2+(aq) + 2 NO 3−(aq) n M= V

Example How many grams of potassium dichromate (K 2 Cr 2 O 7) are required to prepare a 250 m. L solution whose concentration is 2. 16 M?


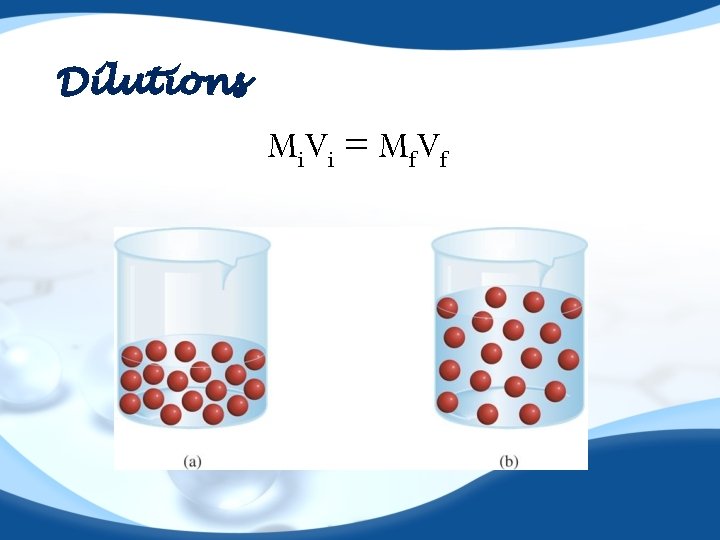
Dilutions M i. V i = M f V f

Example 2 m. L of Describe how you would prepare 5. 00 x 10 a 1. 75 M H 2 SO 4 solution, starting with an 8. 16 M stock solution of H 2 SO 4.

Review of Concepts What is the final concentration of a 0. 6 M Na. Cl solution if its volume is doubled and the number of moles of solute is tripled?

Quantitative analysis • • Gravimetric analysis Titrations • • Acid-base redox

Gravimetric Analysis

Example A 0. 5662 g sample of an ionic compound containing chloride ions and an unknown metal is dissolved in water and treated with and excess of Ag. NO 3. if 1. 0882 g of Ag. Cl precipitate forms, what is the percent by mass of Cl in the original compound?

Acid-base titrations

Example How many m. L of a 0. 610 M Na. OH solution are needed to neutralize 20. 0 m. L of a 0. 245 M H 2 SO 4 solution?


Redox titrations

Example A 16. 42 m. L volume of 0. 1327 M KMn. O 4 solution is needed to oxidize 25. 00 m. L of a Fe. SO 4 solution in an acidic medium. What is the concentration of the Fe. SO 4 solution in molarity? The net ionic equation is 5 Fe 2+ + Mn. O 4− + 8 H+ Mn 2+ + 5 Fe 3+ + 4 H 2 O