PRODUCTION OF METAL POWDERS The selection of materials












































- Slides: 58

PRODUCTION OF METAL POWDERS The selection of materials in powder metallurgy is determined by two factors. i) The alloy required in the finished part. ii) Physical characteristics needed in the powder. iii)Both of these factors are influenced by the process used for making powder.

i) There are numerous ways for powder production which can be categorized as follows. 1) Mechanical methods of powder production: i) Chopping or Cutting ii) Abrasion methods iii) Machining methods iv) Milling v) Cold-stream Process.

2. Chemical methods of powder production: i) Reduction of oxides ii) Precipitation from solutions iii) Thermal decomposition of compounds iv) Hydride decomposition v) Thermit reaction vi) Electro- chemical methods

3. Physical methods of powder production: i) Water atomization ii) Gas atomization iii) Special atomization methods The choice of a specific technique for powder production depends on particle size, shape, microstructure and chemistry of powder and also on the cost of the process.

1. Chopping or Cutting: v In this process, strands of hard steel wire, in diameter as small as 0. 0313 inches are cut up into small pieces by means of a milling cutter. v This technique is actually employed in the manufacturing of cut wire shots which are used for peening or shot cleaning. Limitations: It would, however, be difficult and costly to make powders by this method and for this reason it is not profitable to discuss the technique in detail. 2. Rubbing or Abrasion Methods: These are all sorts of ways in which a mass of metal might be attacked by some form of abrasion. a) Rubbing of Two Surfaces: When we rub two surfaces against each other, hard surface removes the material from the surface of soft material. * Contamination

b) Filing: Filing as a production method has been frequently employed, especially to alloy powders, when supplies from conventional sources have been unobtainable. Such methods are also used for manufacture of coarse powders of dental alloys. Filing can also be used to produce finer powder if its teeth are smaller. * commercially not feasible. c) Scratching: If a hard pin is rubbed on some soft metal the powder flakes are produced. Scratching is a technique actually used on a large scale for the preparation of coarse magnesium powders. * scratching a slab of magnesium with hardened steel pins. * a revolving metal drum to the surface of which is fixed a scratching belt.

The drum, which is about 8 inches in diameter, rotates at a peripheral speed of approximately 2500 ft. /min. The slab of magnesium metal, 14 in. wide by 1. 75 in. thick enters through a gland in the drum casing and presses against the steel pins. d) Machining: A machining process, using for example a lathe or a milling cutter in which something more than just scratching is involved, since the attacking tool actually digs under the surface of the metal and tears it off. On lathe machine by applying small force we get fine chips. A large amount of machining scrap is produced in machining operations. This scrap in the form of chips and turnings can be further reduced in size by grinding. * small scale production.

Disadvantages: • Lack of control on powder characteristics, including chemical contamination such as oxidation, oil and other metal impurities. • The shape of the powder is irregular and coarse. Advantages: • For consuming scrap from another process, machining is a useful process. • Presently the machined powder is used with high carbon steel and some dental amalgam powders.

COMMERCIAL METHODS These are the methods used for high production rate. Best examples of mechanical production methods are the Milling Process and Cold Stream Process. Milling: The basic principal of milling process is the application of impact and shear forces between two materials, a hard and a soft, causing soft material to be ground into fine particles. Milling techniques are suitable for brittle materials. Two types of milling are; i) Ball Milling ii) Attrition Milling.

Objectives of milling include: v. Particle size reduction (comminution or grinding) v. Shape change (flaking v. Solid-state alloying (mechanical alloying) v. Solid-state blending (incomplete alloying) v. Modifying, changing, or altering properties of a material (density, flowability, or work hardening) v. Mixing or blending of two or more materials or mixed phases



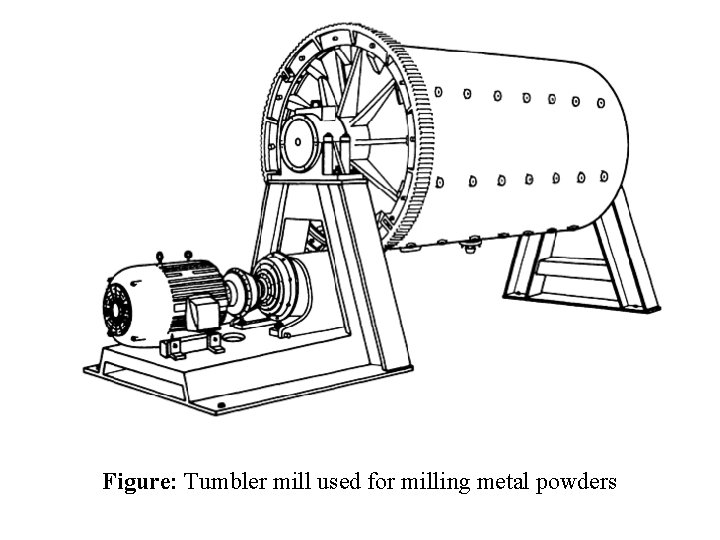
Ball Milling: Ball milling is an old and relatively simple method for grinding large lumps of materials into smaller pieces and powder form. Principle of the process: The principle is simple and is based on the impact and shear forces. Hard balls are used for mechanical comminution of brittle materials and producing powders. Milling Unit: The basic apparatus consists of the following; • A ball mill or jar mill which mainly consists of a rotating drum lined from inside with a hard material. • Hard balls, as a grinding medium, which continue to impact the material inside the drum as it rotates/rolls.


Figure: Tumbler mill used for milling metal powders

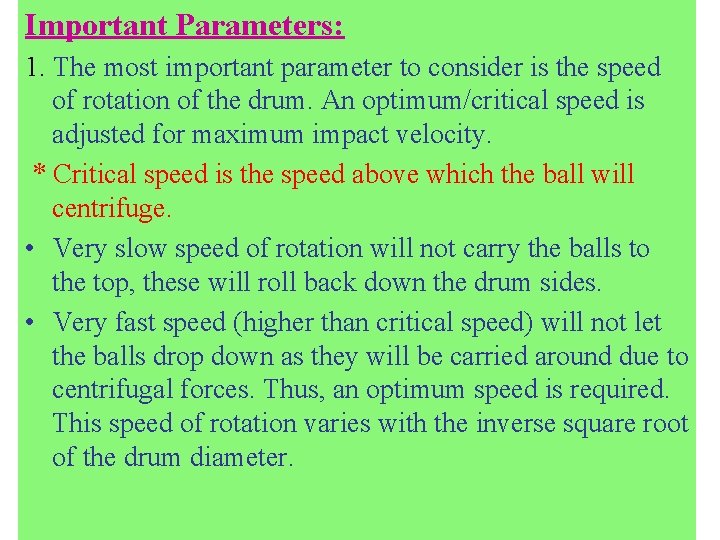
Important Parameters: 1. The most important parameter to consider is the speed of rotation of the drum. An optimum/critical speed is adjusted for maximum impact velocity. * Critical speed is the speed above which the ball will centrifuge. • Very slow speed of rotation will not carry the balls to the top, these will roll back down the drum sides. • Very fast speed (higher than critical speed) will not let the balls drop down as they will be carried around due to centrifugal forces. Thus, an optimum speed is required. This speed of rotation varies with the inverse square root of the drum diameter.

2. The material of grinding media and its size and density. • The size and density of the milling medium is selected according to the deformation and fracture resistance for metals. • For hard and brittle materials large and dense media is used. Whereas, small balls are used for finer grinding. • As a general rule, the balls should be small and their surface should be a little rough. The material of the balls and lining of the drum should be same as that of the material being ground.

3. The rate of milling of a powder is a function of quantity in the total space between the balls. 4. Lubricants and surface active agents are used to nullify the welding forces which causes agglomeration. Grinding Mechanism: During milling the following forces cause fracture of material into powder. Impact Forces: These are caused by instantaneous striking of one object on the other. (Impact is the instantaneous striking of one object by another. Both objects may be moving or one may be stationary). Shear Forces: These are caused as one material slides/rubs against the other.

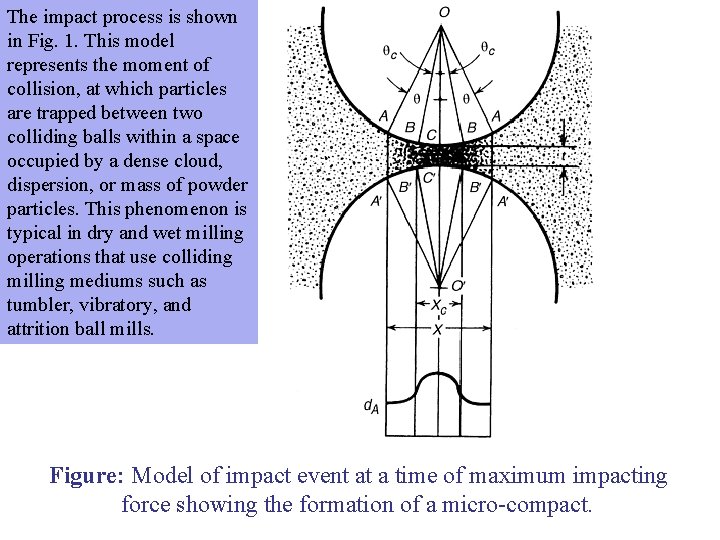
The impact process is shown in Fig. 1. This model represents the moment of collision, at which particles are trapped between two colliding balls within a space occupied by a dense cloud, dispersion, or mass of powder particles. This phenomenon is typical in dry and wet milling operations that use colliding milling mediums such as tumbler, vibratory, and attrition ball mills. Figure: Model of impact event at a time of maximum impacting force showing the formation of a micro-compact.

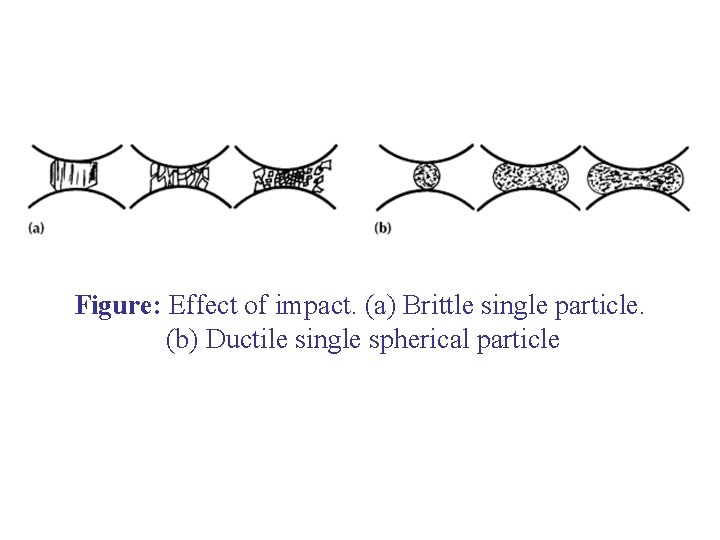
Figure: Effect of impact. (a) Brittle single particle. (b) Ductile single spherical particle

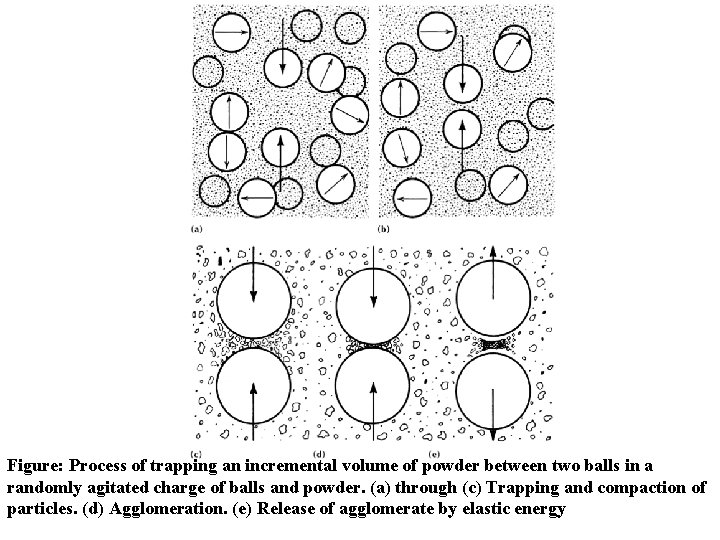
Figure: Process of trapping an incremental volume of powder between two balls in a randomly agitated charge of balls and powder. (a) through (c) Trapping and compaction of particles. (d) Agglomeration. (e) Release of agglomerate by elastic energy

Ø Corrosion of metal in grinding fluid also facilitates comminution. * Ball milling is used for brittle materials. * This method is not suitable for most of the metals due to their ductility and cold welding. Limitations: • Rubbing action causes contamination of powder since balls may also get rubbed. • Working hardening of metal powder is caused during milling. • There is a possibility of excessive oxidation of final powder. • Quality of powder is poor. • Particle welding and agglomeration may take place.

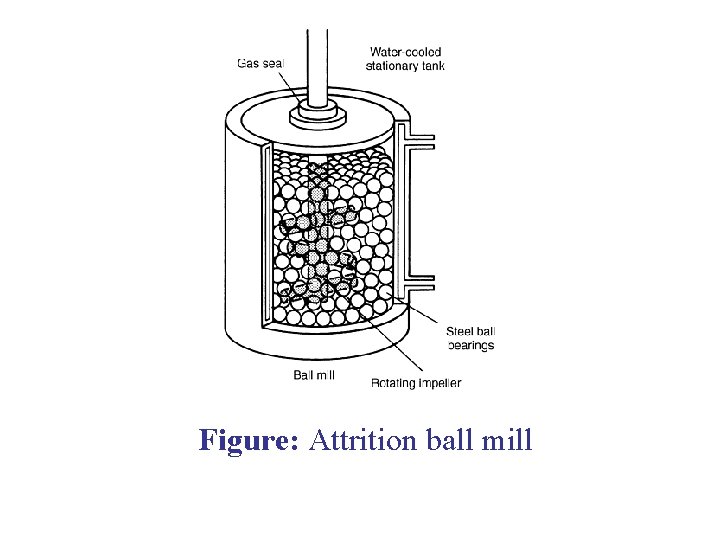
ATTRITION MILLING Attrition is the term which means to wear or rub away. It is a process of grinding down by friction. Milling Unit: • In attrition milling a very high efficiency ball mill is agitated by a vertical rotating shaft with horizontal arms. • In these mills the rotational speeds are nearly 6 – 80 rpm while the size of medium (balls) used is 3 – 6 mm. • Power is used to rotate the agitator and not the vessel as in case of ball mills. The central rotating shaft of attrition mill is equipped with several horizontal arms. When rotated, it exerts the stirring action to tumble the grinding medium randomly throughout the entire chamber.



Mechanism of milling: • The milling action is done by impact and shear forces. The charge is impacted by balls traveling in various trajectories that collide within the area. • Impaction is caused by constant impinging of grinding medium due to irregular movements. • Shearing action is produced by random movement of balls in different rotational directions which exert shearing force on adjacent slurry. * Continuous attrition mills • Powders of very hard materials such as ceramics, carbides and hard metals are being produced by this technique. • The particle size becomes finer with increasing milling time and the shape of particle is angular. • To avoid possible contamination, the balls, stirring rods and the tank may be made from same material as the powder.

Figure: Attrition ball mill

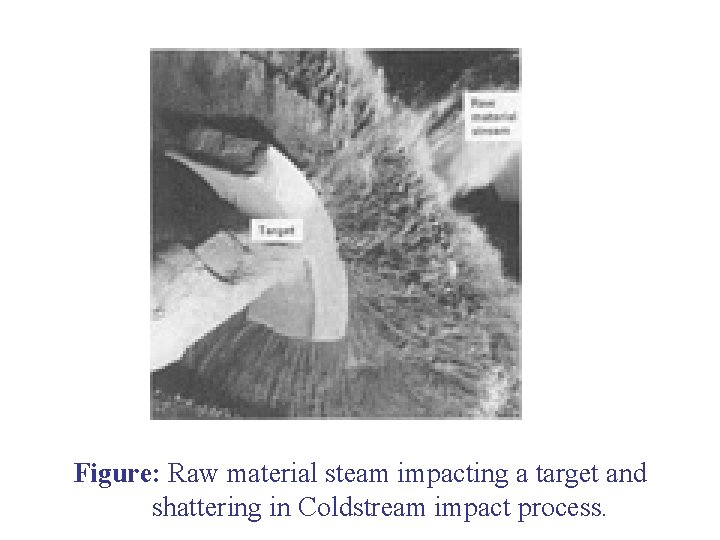
COLD STREAM PROCESS • This process is based on impact phenomenon caused by impingement of high velocity particles against a cemented carbide plate. • The unit consists of: Ø A feed container; Ø A compressor capable of producing a high velocity stream of air (56 m 3/min. ) operating at 7 MPa (1000 psi); Ø A target plate, made of cemented tungsten carbide, for producing impact; Ø A classifying chamber lined with WC while the supersonic nozzle and target generally are made of cemented tungsten carbide.

Mechanism of the Process: The material to be powdered is fed in the chamber and from there falls in front of high velocity stream of air. This air causes the impingement of material against target plate, where material due to impaction is shattered into powder form. This powder is sucked and is classified in the classifying chamber. Oversize is recycled and fine powder is removed from discharge area. * Rapidly expanding gases leaving the nozzle create a strong cooling effect through adiabatic expansion. This effect is greater than the heat produced by pulverization.

Figure: Raw material steam impacting a target and shattering in Coldstream impact process.

CHEMICAL METHODS • Almost all metallic elements can be produced in the form of powders by suitable chemical reactions or decomposition. • For example all chemical compounds can be decomposed into their elements if heated to sufficient high temperatures. • If the non-metallic radical could be removed, for example by continuous evacuation or by entrainment in an inert gas, then practical methods of making metal powders might be feasible.

Theory of the process: Mostly chemical methods are based on the decomposition of a compound into the elemental form with heating or with the help of some catalyst. In most cases such processes involve at least two reactants. (i) a compound of the metal (ii) a reducing agent

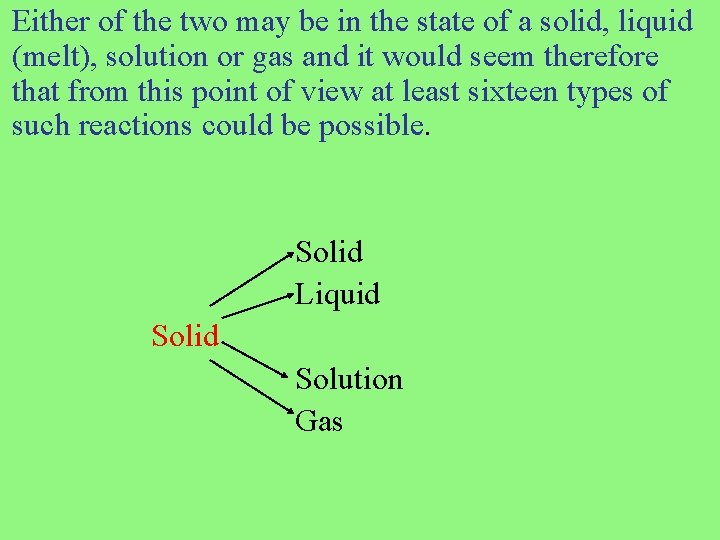
Either of the two may be in the state of a solid, liquid (melt), solution or gas and it would seem therefore that from this point of view at least sixteen types of such reactions could be possible. Solid Liquid Solution Gas

The chemical processes can be discussed under the headings of: (i) Decomposition of solid phases. (ii) Precipitation of Aqueous Solutions (iii) Precipitation from Melts (iv) Decomposition of Gaseous Phases

Classification of Chemical Methods: The well known techniques which are based on chemical/thermal decomposition are; (i) Reduction of oxides (ii) Precipitation from solutions (iii) Thermal decomposition (iv) Hydride decomposition (v) Thermite reaction (vi) Electro-chemical method

REDUCTION OF METAL OXIDES Manufacturing of metal powder by reduction of oxides is extensively employed, particularly for Fe, Cu, W and Mo. As a manufacturing technique, oxide reduction may exhibit certain advantages and disadvantages. These are listed below; Advantages: ØA variety of reducing agents can be used and process can be economical when carbon is used. ØClose control over particle size --- because oxides are generally friable, easily pulverized and easily graded by sieving. ØPorous powders can be produced which have good compressive properties. ØAdoptability either to very small or large manufacturing units and either batch or continuous processes.

Limitations: v. Process may be costly if reducing agents are gases. v. Large volumes of reducing gas may be required, and circumstances where this is economically available may be limited; in some cases, however, costs may be reduced by recirculation of the gas. v. The purity of the finished product usually depends entirely upon the purity of the raw material, and economic or technical considerations may set a limitation to that which can be attained. v. Alloy powders cannot be produced.

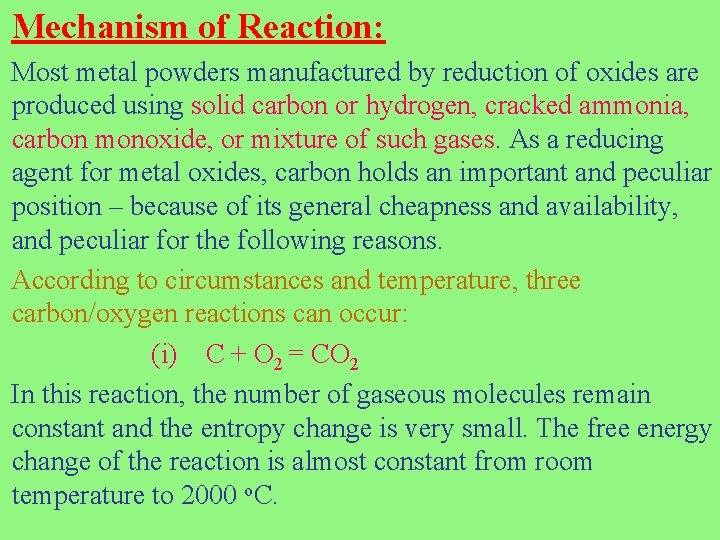
Mechanism of Reaction: Most metal powders manufactured by reduction of oxides are produced using solid carbon or hydrogen, cracked ammonia, carbon monoxide, or mixture of such gases. As a reducing agent for metal oxides, carbon holds an important and peculiar position – because of its general cheapness and availability, and peculiar for the following reasons. According to circumstances and temperature, three carbon/oxygen reactions can occur: (i) C + O 2 = CO 2 In this reaction, the number of gaseous molecules remain constant and the entropy change is very small. The free energy change of the reaction is almost constant from room temperature to 2000 o. C.

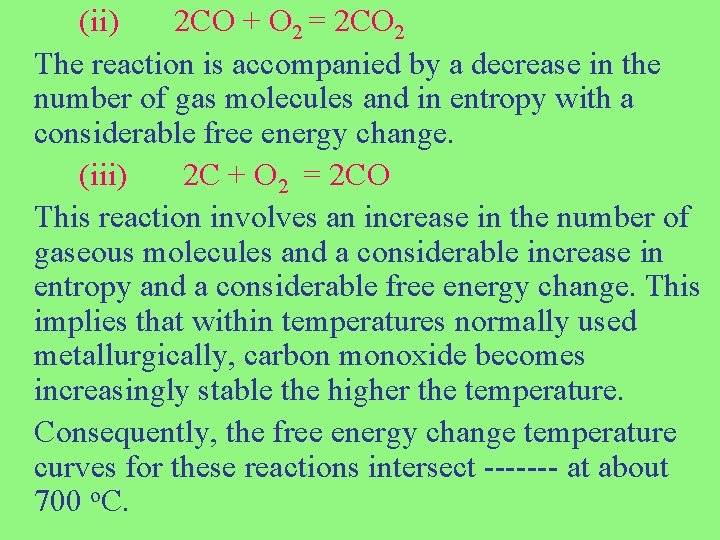
(ii) 2 CO + O 2 = 2 CO 2 The reaction is accompanied by a decrease in the number of gas molecules and in entropy with a considerable free energy change. (iii) 2 C + O 2 = 2 CO This reaction involves an increase in the number of gaseous molecules and a considerable increase in entropy and a considerable free energy change. This implies that within temperatures normally used metallurgically, carbon monoxide becomes increasingly stable the higher the temperature. Consequently, the free energy change temperature curves for these reactions intersect ------- at about 700 o. C.

The important implication of these facts is that, (a) All metal oxide are reducible by carbon from very low to very high temperatures ------ although practically the temperatures necessary may be too high, but (b) The reaction must be prevented from reversing on cooling, and (c) The product of the reduction will be mainly CO 2 below 700 o. C and mainly CO above this temperature. At high temperatures, any carbon dioxide is reduced by any excess carbon, forming more stable CO.

(a) (b) (c) (d) When using a reducing gas, continued contact between the oxide and the reducing gas must take place by; Diffusion of gas through the metal to the oxide, Diffusion of oxygen, or oxide, through the metal to the gas, Both (a) and (b), or Movement of one kind or another through pores.

Production of Iron Powder by Reduction of Iron Oxide: (Direct Reduction Process) Iron powders are commercially used for a large number of applications such as fabrication of structural parts, welding rods, flame cutting, food enrichment and electronic and magnetic applications. The classical technique for production of iron powder is the reduction of iron oxide.

Theory of the process: It is the oldest process of production of iron powder by using carbon as the reducing agent. In this process pure magnetite (Fe 3 O 4) is used. Coke breeze is the carbon source used to reduce iron oxide. Some limestone is also used to react with the sulphur present in the coke. The mixture of coke and limestone (85% + 15%) is dried in a rotary kiln and crushed to uniform size. ** Hoganas Process

The ore and coke-limestone mixture is charged into ceramic tubes (Silicon Carbide) with care so that ore and reduction mixture are in contact with each other but not intermixed. It can be achieved by using concentric charging tubes with in the ceramic tube. (A pair of concentric steel charging tubes is lowered to the bottom of the ceramic tubes. The ore is fed between the steel tubes. The coke-limestone mixture is fed within the inner of the two concentric charging tubes and between the outer charging tube and the inner wall of the ceramic tube, leaving the ore and the reduction mixture in contact with one another, but not intermixed. )

Charged ceramic tubes are loaded on the Kiln cars (thirty six tubes on each) and cars are pushed into 170 meter long tunnel kiln where the reduction occurs. The total time a car is present in the kiln is 68 hrs. Gas burners heat the 150 meter tunnel at a temperature of 1200 -1260 o. C and remaining length is cooled by air circulation. Within the hot zone, several chemical reactions occur and metallic iron is formed in the form of sponge cake. The main reaction is; MO + R M + RO
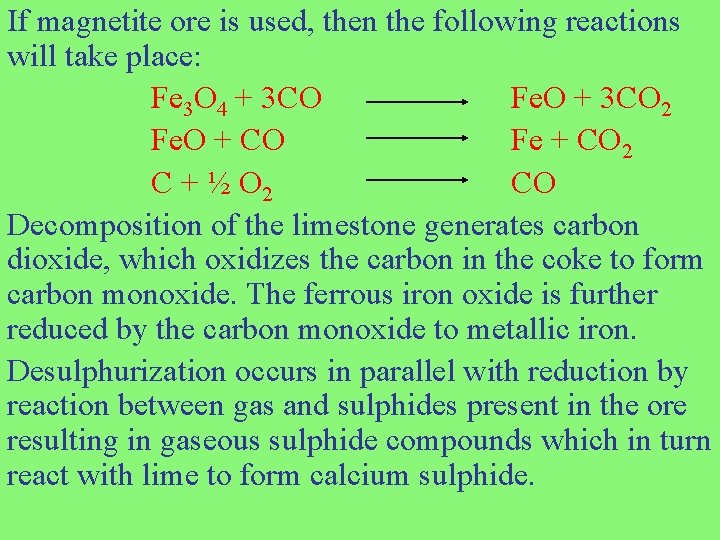
If magnetite ore is used, then the following reactions will take place: Fe 3 O 4 + 3 CO Fe. O + 3 CO 2 Fe. O + CO Fe + CO 2 C + ½ O 2 CO Decomposition of the limestone generates carbon dioxide, which oxidizes the carbon in the coke to form carbon monoxide. The ferrous iron oxide is further reduced by the carbon monoxide to metallic iron. Desulphurization occurs in parallel with reduction by reaction between gas and sulphides present in the ore resulting in gaseous sulphide compounds which in turn react with lime to form calcium sulphide.

The sponge cake is removed from ceramic tubes and dropped into a tooth crusher where this is broken into pieces. After these pieces are ground to desired particle size. During grinding the powder particles are considerably work hardened. The powder is annealed at 800 - 870 o. C in the atmosphere of dissociated ammonia. The powder is loosely sintered, but requires only light grinding and screening to produce a finished product.

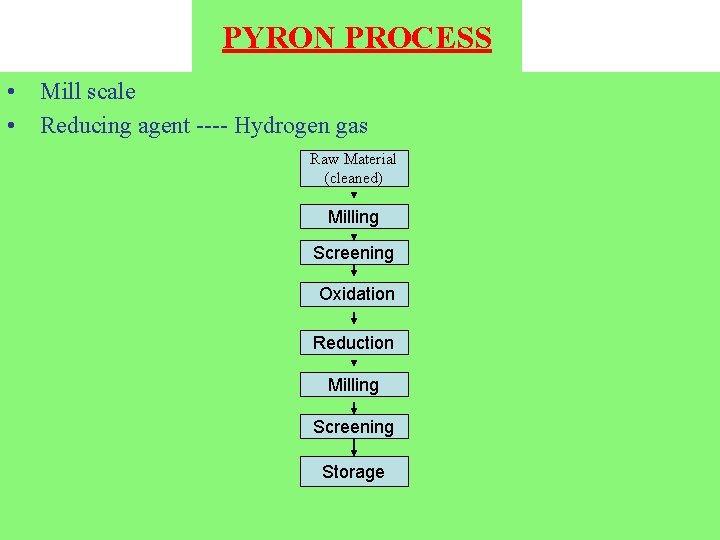
PYRON PROCESS • Mill scale • Reducing agent ---- Hydrogen gas Raw Material (cleaned) Milling Screening Oxidation Reduction Milling Screening Storage

• Mill scale is basically obtained from steel mills which produce sheets, rods, wires, plates and pipes. • The mill scale mainly consists of Fe 3 O 4, and also contains oxides of tramp elements normally associated with steel, especially Si, Mn and Cr in the form of very finely dispersed oxides ----- difficult to reduce. • The mill scale is dried and ground up to the desired particle size in a continuous ball mill. (- 100 mesh) • Oxidation of the mill scale at 870 to 980 o. C converts Fe O and Fe 3 O 4 to ferric oxide (Fe 2 O 3). This process is essential to ensure uniform properties of Pyron-iron Powder.

• Reduction of ferric oxide by hydrogen is done in an electric furnace (30 – 40 meter long) at 980 o. C. (continuous belt furnace). • Hydrogen is supplied by NH 3 cracking plant and reduction is done at 980 o. C. Fe 2 O 3 + 3 H 2 2 Fe+3 H 2 O • The reduction product is ground and mechanically densified to make it suitable for production of structural parts. • Fine particle size -----small pores ------faster sintering.

Powder Characteristics: • The Pyron Powder is a porous and finer. • It has sponge like microstructure. • It sinters faster as compared to powder formed by other commercials processes. Advantages: Ø There is no relative movement of particles of the charge to each other or to the belt, therefore sticking and welding is avoided. Ø Low carbon contents in the final product because of hydrogen. Ø Low labor cost. Ø Thin beds and continuous flow of reducing gases lead to a comparatively short time of reduction. * The purity of the iron powder product is entirely a function of the raw mill scale.

HYDRIDE DECOMPOSITION This method of powder production is used for precious metals. Hydrides are binary compounds of metals and hydrogen. The main steps are as follows: (i) Hydride Formation: In this step turnings of metals (Ti, U, Zr etc) are heated in hydrogen resulting in the formation of hydrides. (ii) Milling: Hydrides are brittle in nature and thus can be easily crushed and ground to fine powder. (iii) Dehydridation: The fine powder of hydrides is heated under vacuum at elevated temperature to eliminate hydrogen from metal, and consequently a fine metal powder is obtained.

PRECIPITATION FROM SOLUTIONS • • This method is used for precious metals. Leaching an ore or ore concentrate, followed by precipitating the metal from leach solution. Steps Involved: i) Formation of insoluble compounds/precipitates: The salts of metals are converted/precipitated as insoluble hydroxides, carbonates or oxalates etc. ii) Decomposition: On heating, these compounds/ppts. decompose into metal or metal oxides and gaseous products. *The examples of this technique are the production of uranium dioxide, platinum, selenium, silver, nickel and cadmium oxides.

Powder characteristics: Ø The chemically precipitated powders can have high purity and have fine particle size and tendency towards agglomeration. Ø The particle shape is irregular or cubic or sometime it is sponge like. Ø The flow properties of these powders are poor and the packing densities are low.

**In some cases, powder is produced by gaseous reactions, i. e. metal chlorides, fluorides or oxides of vanadium, niobium, tungsten, uranium, titanium, and zirconium are reduced with sodium, magnesium or hydrogen. The reaction product is leached with dilute hydrochloric acid to remove sodium and magnesium chlorides. The resulting powder is spongy like with irregular shape.

THE CARBONYL PROCESS • The only method for the manufacture of metal powder by the pyrolysis of a gaseous compound which has been used industrially on a substantial scale is the carbonyl iron or nickel process. • When iron and nickel ores react under high pressure (70 – 300 atm. ) with carbon monoxide, iron pentacarbonyl [Fe(CO)5] or nickel tetracarbonyl [Ni(CO)4] is formed, respectively. • Both compounds are liquids at room temperature. • Fe(CO)5 evaporates at 103 o. C and Ni(CO)4 at 43 o. C.

Precipitate Formation: This step of the process is carried out according to the following scheme: Ø The liquid carbonyles are stored under pressure in tanks submerged in water. Ø The distilled and filtered liquids are conveyed to steam heating cylinders, where they are vaporized. Ø The vapors of liquid are sent to decomposers. The decomposers are jacketed and heated, giving an internal temperature of 200 – 250 o. C. These cylinders are 9 – 10 feet high with an internal dia of 3 feet, with conical bottoms. Ø The incoming stream of vapors meets a tangential stream of ammonia gas. CO is removed here and precipitates of metals are formed which are then sieved, dried and may be milled to break up the agglomerates. Ø The CO gas arising from the decomposition is recovered and re-used.

v. Carbonyl iron powder is used for the production of magnetic powder cores for radio or television applications. v. In P/M it is used for the manufacture of soft magnetic materials and permanent magnets. v. Because of its high price and poor die filling properties, it is not suitable for the manufacture of sintered structural components. v. The carbonyl process is also well suited for the extraction of both metals from lean ores. The process can be controlled so as to yield a spherical metal powder.
 Dusting powder definition in pharmacy
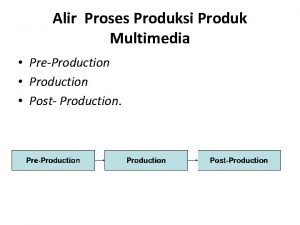
Dusting powder definition in pharmacy Sebutkan 3 proses produksi multimedia
Sebutkan 3 proses produksi multimedia States of matter venn diagram
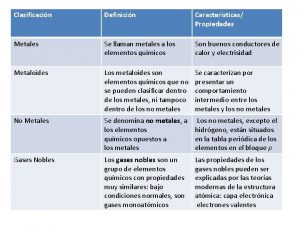
States of matter venn diagram Metals vs nonmetals properties
Metals vs nonmetals properties Acidity trends periodic table
Acidity trends periodic table Melting point of diamond
Melting point of diamond Pmos nwell
Pmos nwell Uses non metals
Uses non metals Properties of semimetals
Properties of semimetals When a metal reacts with a nonmetal the metal will
When a metal reacts with a nonmetal the metal will Metal no metal y metaloide tabla periodica
Metal no metal y metaloide tabla periodica Blanch def
Blanch def Propiedades y características de los elementos
Propiedades y características de los elementos Example of metal
Example of metal El sodio es metal o no metal
El sodio es metal o no metal Balancing selection vs stabilizing selection
Balancing selection vs stabilizing selection Similarities
Similarities K selection r selection
K selection r selection Natural selection vs artificial selection
Natural selection vs artificial selection Artificial selection vs natural selection
Artificial selection vs natural selection Naturual selection
Naturual selection K selection r selection
K selection r selection Natural selection vs artificial selection
Natural selection vs artificial selection Two way selection and multiway selection in c
Two way selection and multiway selection in c Multiway selection
Multiway selection Mass selection and pure line selection
Mass selection and pure line selection Factors considered for selection of materials
Factors considered for selection of materials Raw materials quality control
Raw materials quality control Natural materials
Natural materials What are the useful materials at home?
What are the useful materials at home? Man made map
Man made map Adapting and adopting materials
Adapting and adopting materials Direct materials budget with multiple materials
Direct materials budget with multiple materials How to mix powders homogeneously
How to mix powders homogeneously Dusting powder examples
Dusting powder examples Density of milk
Density of milk Eutectic mixture slideshare
Eutectic mixture slideshare What are powders
What are powders Mystery powders lab
Mystery powders lab Tumbling in pharmacy
Tumbling in pharmacy Flow properties of pharmaceutical powders
Flow properties of pharmaceutical powders Define hausner ratio
Define hausner ratio Granulés effervescents
Granulés effervescents Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Glasgow thang điểm
Glasgow thang điểm Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới