Power Point Presentation to Accompany 2010 Delmar Cengage












- Slides: 35

Power. Point Presentation to Accompany © 2010 Delmar, Cengage Learning

Chapter 2 The Chemistry of Life © 2010 Delmar, Cengage Learning 2

Introduction • Cells, tissues and organs composed of chemicals • Chemical reactions important for function • Chemistry is the study of: – Elements, compounds, chemical reactions, molecular structure © 2010 Delmar, Cengage Learning 3

Atomic Structure © 2010 Delmar, Cengage Learning 4

Atomic Structure (cont’d. ) • Atoms – Smallest particles of elements – Maintain all characteristics of element – Nucleus contains protons and neutrons – Electrons orbit nucleus in shells © 2010 Delmar, Cengage Learning 5

Elements, Isotopes, Compounds © 2010 Delmar, Cengage Learning 6

Elements, Isotopes, Compounds (cont’d. ) • Element: atoms contain same numbers of protons and electrons • Compound: contains two or more elements • Isotope: number of neutrons varies • Periodic table of the elements – Arranges elements by increasing atomic number © 2010 Delmar, Cengage Learning 7

Elements, Isotopes, Compounds (cont’d. ) • Orbital: area where electron is found • Energy levels: grouping of orbitals – Represented as concentric circles surrounding nucleus © 2010 Delmar, Cengage Learning 8

Bonds and Energy © 2010 Delmar, Cengage Learning 9

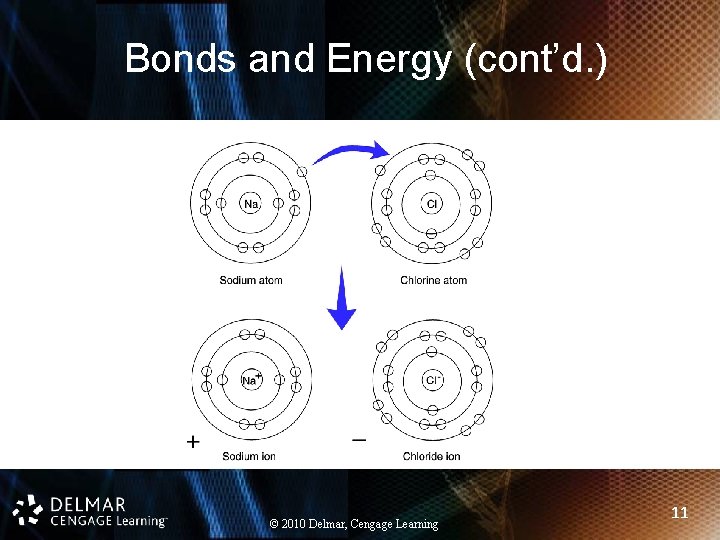
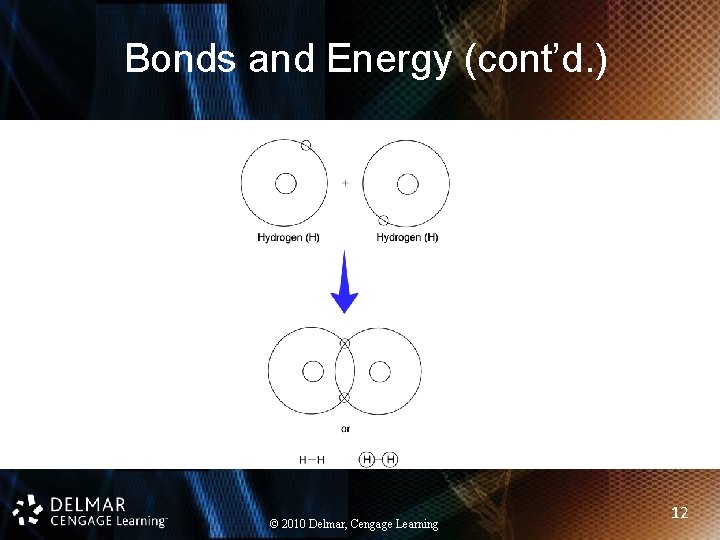
Bonds and Energy (cont’d. ) • Ionic: one atom gains and one atom loses electrons • Covalent: atoms share electrons • Hydrogen: weak bonds, hold water molecules together • Electron donors vs. acceptors vs. carriers • Bonds are energy containing © 2010 Delmar, Cengage Learning 10

Bonds and Energy (cont’d. ) © 2010 Delmar, Cengage Learning 11

Bonds and Energy (cont’d. ) © 2010 Delmar, Cengage Learning 12

Common Substances in Living Systems © 2010 Delmar, Cengage Learning 13

Water • • • Most abundant substance in cells Universal solvent Transport of materials Absorbs and reduces heat Protects body structures © 2010 Delmar, Cengage Learning 14

Carbon Dioxide • Waste product of cellular respiration • Used in photosynthesis to produce usable energy sources • Must be removed quickly from cells • Carbon in molecules comes from carbon dioxide gas © 2010 Delmar, Cengage Learning 15

Molecular Oxygen • Formed from covalent bond of two oxygen atoms • Required by all organisms that breathe air • Necessary to convert food into ATP • Level in atmosphere is 21% © 2010 Delmar, Cengage Learning 16

Ammonia • By-product of amino acid breakdown – Amino acids are building blocks of proteins – Amino acids contain nitrogen • Converted to urea in the liver © 2010 Delmar, Cengage Learning 17

Mineral Salts • Composed of small ions • Calcium: muscle contraction and strong bones • Phosphate - ATP synthesis • Sodium, potassium, and chloride are necessary for muscle contraction and nervous transmission © 2010 Delmar, Cengage Learning 18

Carbohydrates • 1: 2: 1 ratio of carbon, hydrogen and oxygen • Five- and six-carbon simple sugars are smallest – Five-carbon: deoxyribose and ribose – Six-carbon: glucose and fructose • Functions: energy storage and cell structure © 2010 Delmar, Cengage Learning 19

Lipids • Insoluble in water • 95% of fats in body are triacylglycerols • Saturated fat: fatty acids have single covalent bonds • Unsaturated fat: fatty acids have one or more double covalent bonds • Functions: energy, insulation and protection © 2010 Delmar, Cengage Learning 20

Proteins • Contain carbon, oxygen, hydrogen, nitrogen and sulfur • Amino acids are building blocks of proteins • Functions: energy and structure • Enzymes: protein catalysts for chemical reactions © 2010 Delmar, Cengage Learning 21

Proteins (cont’d. ) • Structure – Primary: amino acid sequence – Secondary: determined by hydrogen bonds – Tertiary: folding caused by interactions within peptide bonds and sulfur atoms – Quaternary: determined by spatial relationships between units © 2010 Delmar, Cengage Learning 22

Nucleic Acids • Deoxyribonucleic acid: genetic material of the cell • Ribonucleic acid: protein synthesis – Messenger RNA – Transfer RNA • Structure – DNA: double helical chain – RNA: single chain © 2010 Delmar, Cengage Learning 23

Nucleic Acids (cont’d. ) • Nucleic acids are made up of chains of nucleotides – Nucleotide: nitrogen base, sugar and phosphate group – Nitrogen bases: purines (two) and pyrimidines (three) © 2010 Delmar, Cengage Learning 24

Adenosine Triphosphate • Fuel for cell function and maintenance • Molecule consists of sugar, adenine, and three phosphates – Energy is stored in the second and third phosphates • Breakdown of glucose provides energy to make ATP © 2010 Delmar, Cengage Learning 25

Movement of Materials Into and Out of Cells © 2010 Delmar, Cengage Learning 26

Introduction • Plasma membrane is selectively permeable – Only selected materials can enter and exit – This is because of chemical structure – Water can enter and exit with ease © 2010 Delmar, Cengage Learning 27

Diffusion • Movement of molecules from area of high concentration to low concentration • Brownian movement: random collision of diffusing molecules • Accelerated by increased temperature • O 2 - CO 2 exchange is an example of diffusion © 2010 Delmar, Cengage Learning 28

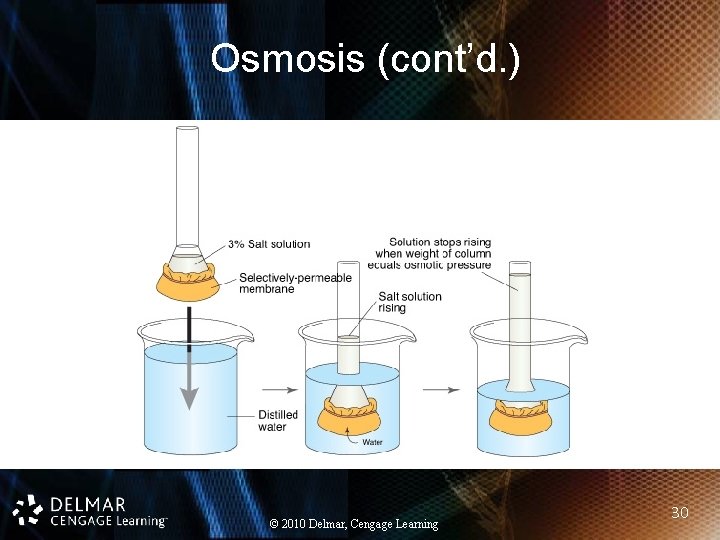
Osmosis • Movement of water through semipermeable membrane from high concentration to low concentration – Isotonic solution: salt concentration is the same outside the cell as inside – Hypotonic solution: salt concentration inside cell is higher than outside cell – Hypertonic solution: salt concentration higher outside the cell than inside © 2010 Delmar, Cengage Learning 29

Osmosis (cont’d. ) © 2010 Delmar, Cengage Learning 30

Osmosis (cont’d. ) • Active transport – Used by cells to obtain sugars, amino acids, larger proteins and fats – Needs energy in the form of ATP – Molecules move from areas of low concentration to areas of high concentration © 2010 Delmar, Cengage Learning 31

p. H © 2010 Delmar, Cengage Learning 32

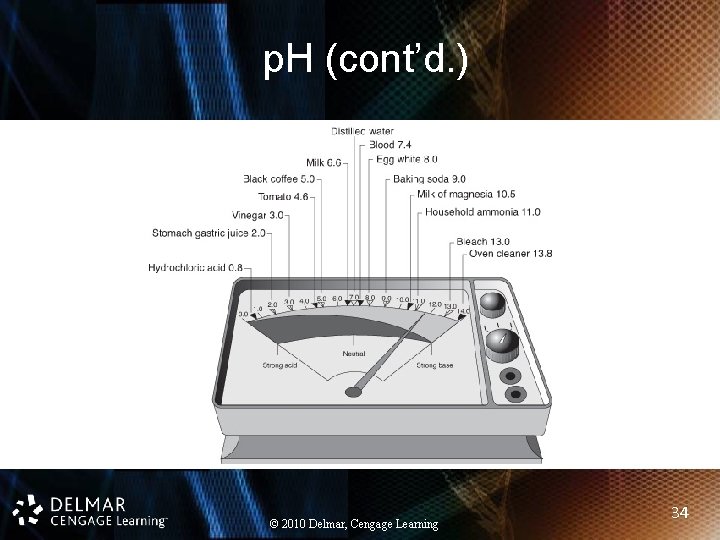
p. H (cont’d. ) • Negative logarithm of hydrogen ion concentration • Acids: p. H values below 7 • Bases: p. H values above 7 • Buffers: help maintain p. H levels © 2010 Delmar, Cengage Learning 33

p. H (cont’d. ) © 2010 Delmar, Cengage Learning 34

Summary • Discussed basic chemical concepts such as bonds and energy and how they apply to living systems • Discussed specific chemical substances and how they are used in living systems • Described three ways that substances move into and out of cells • Introduced p. H and acids/bases © 2010 Delmar, Cengage Learning 35
 Chapter 6:2 interpreting word parts
Chapter 6:2 interpreting word parts 2009 delmar cengage learning
2009 delmar cengage learning Challenge word building medical terminology
Challenge word building medical terminology Measuring and recording apical pulse
Measuring and recording apical pulse 2009 delmar cengage learning
2009 delmar cengage learning Chapter 13 medical math assignment sheet
Chapter 13 medical math assignment sheet 2009 delmar cengage learning
2009 delmar cengage learning Delmar cengage learning instructor resources
Delmar cengage learning instructor resources 2010 cengage learning
2010 cengage learning Hebrews 6 9
Hebrews 6 9 Accompany chapter 1
Accompany chapter 1 Venous supply of upper limb
Venous supply of upper limb Printers create objects such as prototypes and models.
Printers create objects such as prototypes and models. Microsoft power point 2010
Microsoft power point 2010 Subject and topic in hindi
Subject and topic in hindi Power point presentation design west vancouver
Power point presentation design west vancouver Delmar isotonic
Delmar isotonic Delmar tsi
Delmar tsi Delmar customs broker
Delmar customs broker Delmar thomson learning
Delmar thomson learning Delmar larsen
Delmar larsen Power triangle
Power triangle Power bi power point
Power bi power point Point point power
Point point power Chapter 7 cengage
Chapter 7 cengage Cengage differential equations
Cengage differential equations Century 21 bank
Century 21 bank Separation of variables differential equations
Separation of variables differential equations Cengage chapter 7
Cengage chapter 7 Medical terminology chapter 5 learning exercises answers
Medical terminology chapter 5 learning exercises answers Cengage learning heart diagram
Cengage learning heart diagram Cengage
Cengage Cengage
Cengage Cengage
Cengage South-western cengage learning
South-western cengage learning Cengage
Cengage