Petrochemicals 1 Petrochemical Industry Feedstocks for petrochemicals are






- Slides: 40

Petrochemicals 1

Petrochemical Industry Ø Feedstocks for petrochemicals are gas and light to middle petroleum liquids Ø Natural gas can also be used as feedstock for petrochemicals ØNearly all petrochemicals are produced over catalysts Ø Both homogeneous and heterogeneous catalysts are involved 2

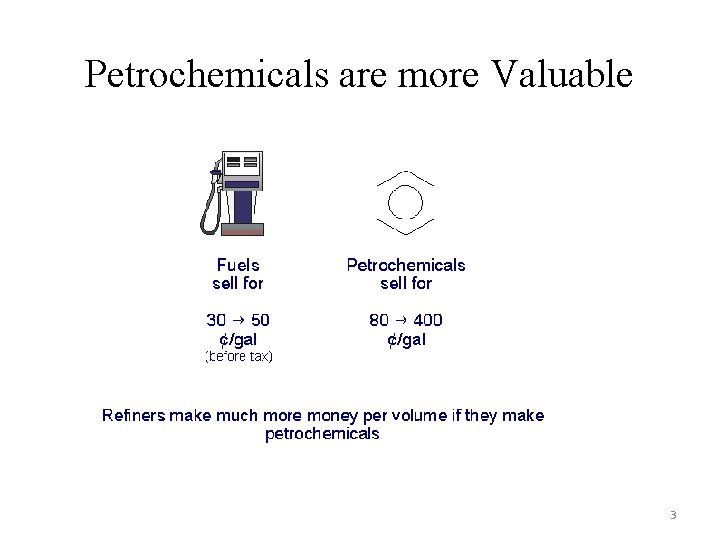
Petrochemicals are more Valuable 3

The big seven hydrocarbons: Methane 4

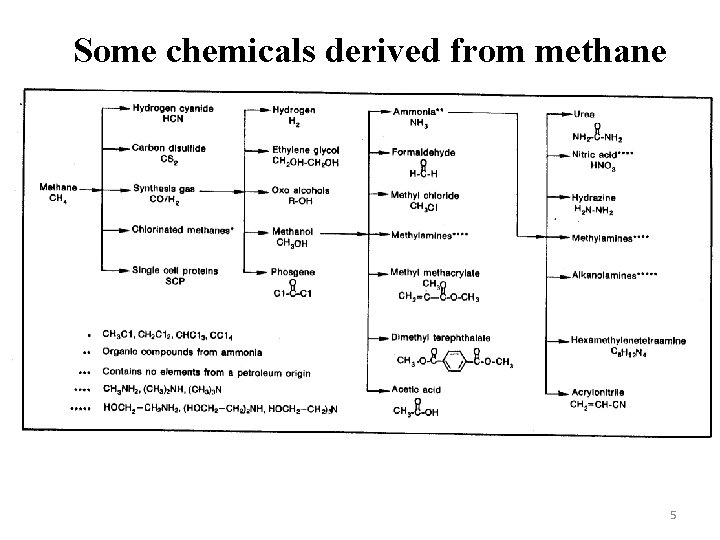
Some chemicals derived from methane 5

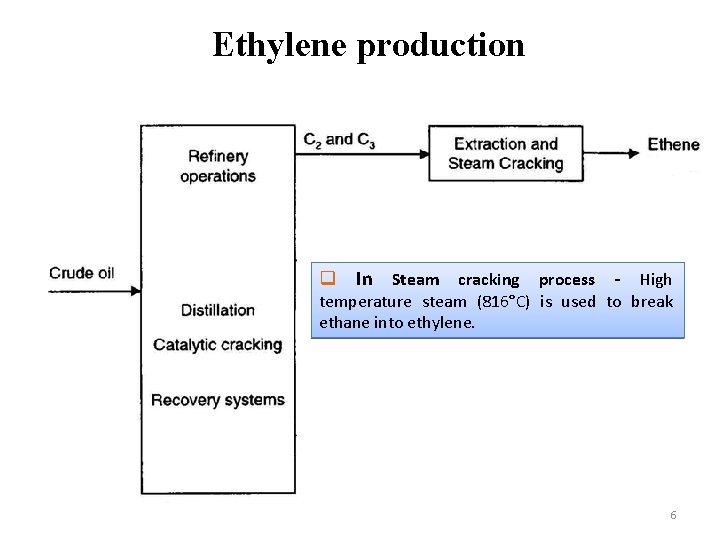
Ethylene production q In Steam cracking process - High temperature steam (816°C) is used to break ethane into ethylene. 6

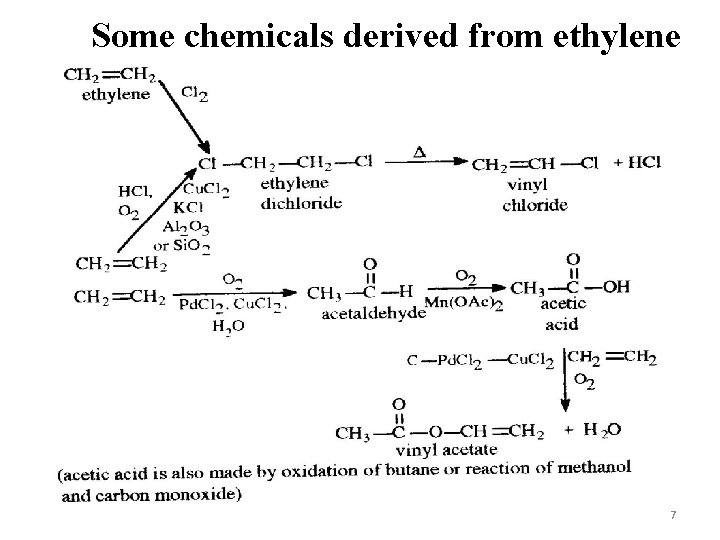
Some chemicals derived from ethylene 7

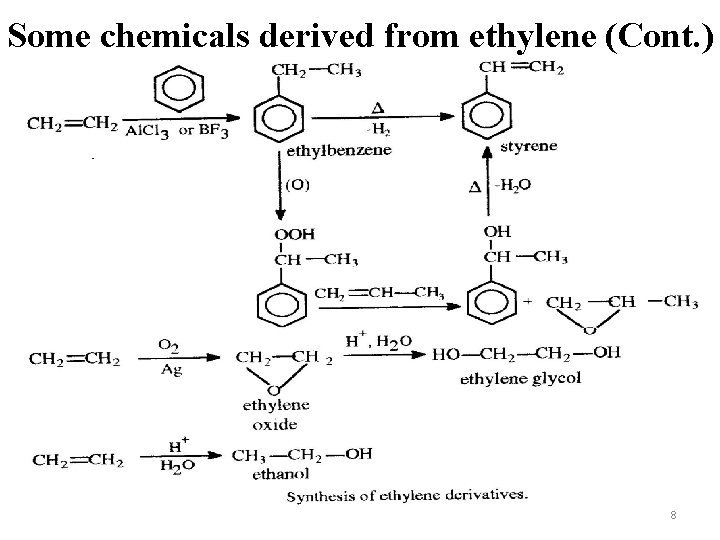
Some chemicals derived from ethylene (Cont. ) SMPO process P 164 -2 8

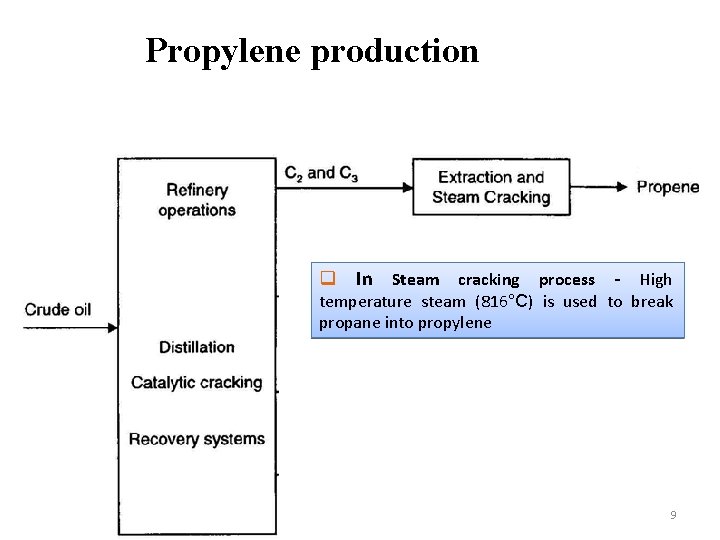
Propylene production q In Steam cracking process - High temperature steam (816°C) is used to break propane into propylene 9

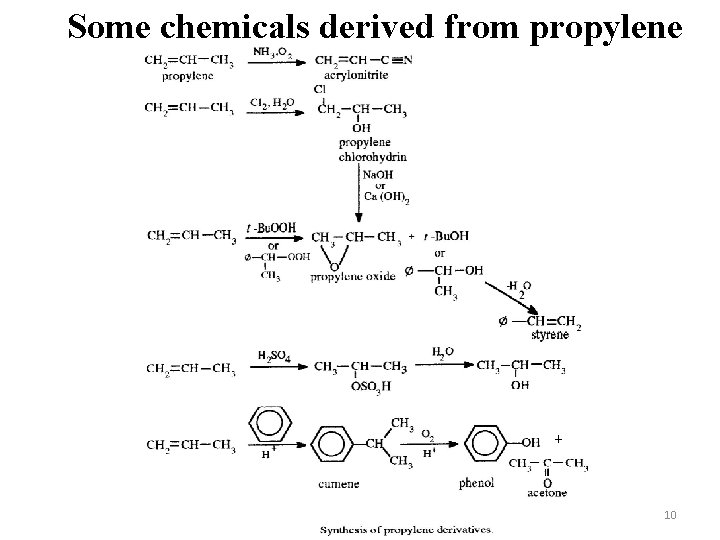
Some chemicals derived from propylene + 10

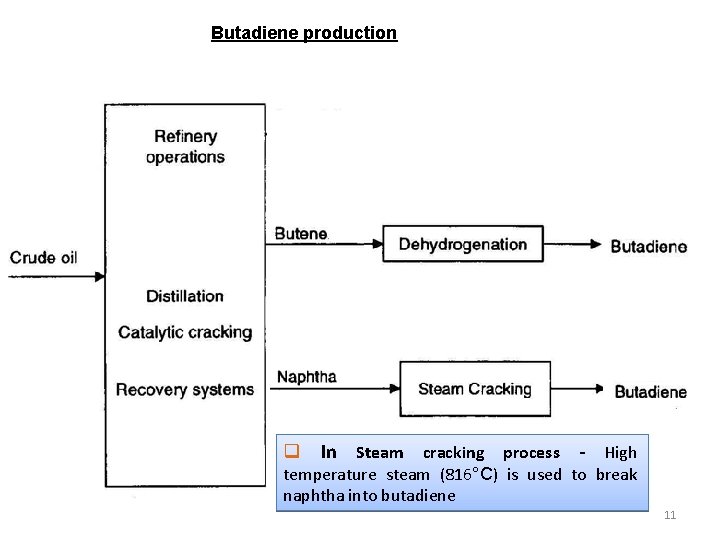
Butadiene production q In Steam cracking process - High temperature steam (816°C) is used to break naphtha into butadiene 11

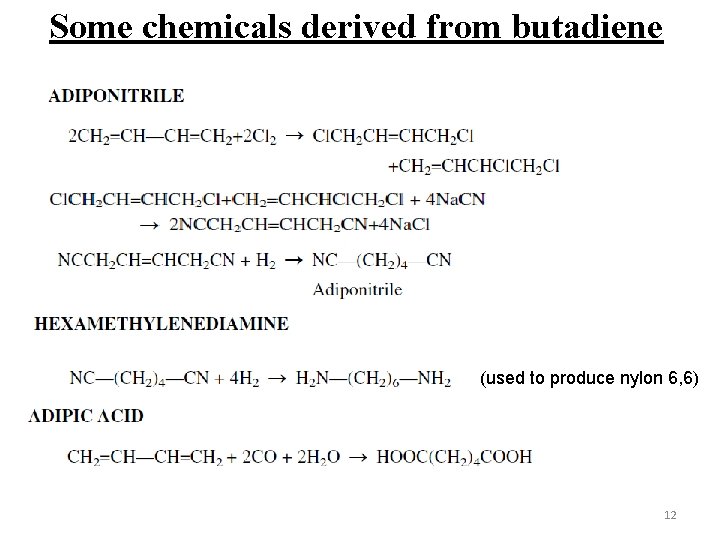
Some chemicals derived from butadiene (used to produce nylon 6, 6) 12

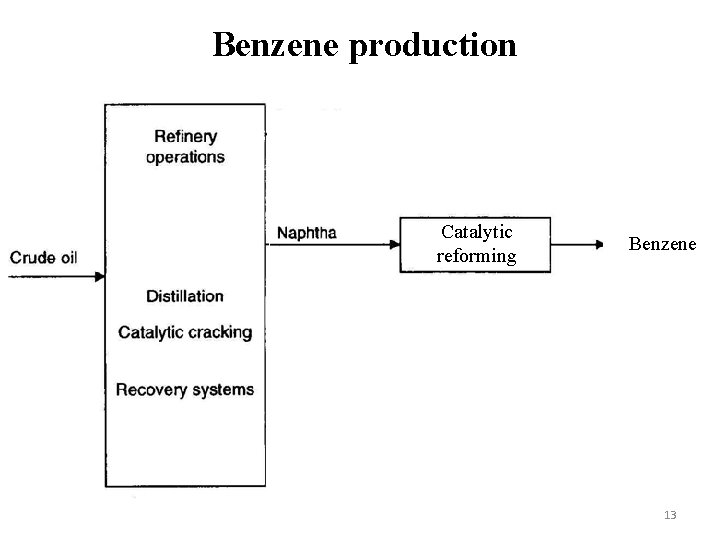
Benzene production Catalytic reforming Benzene 13

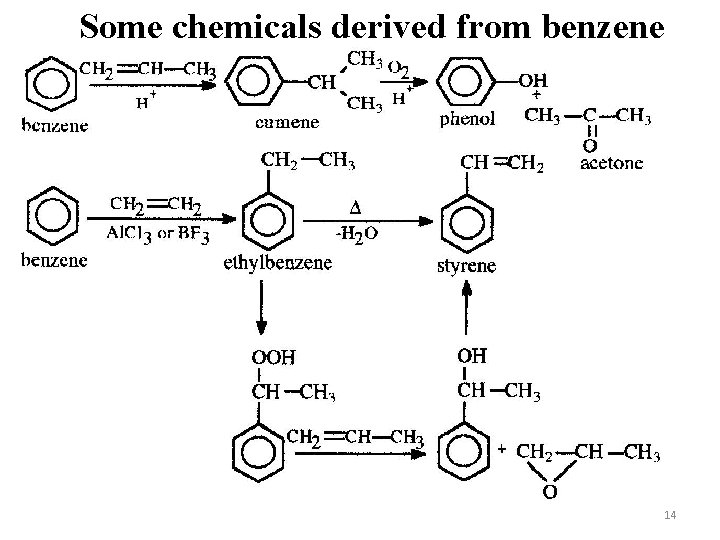
Some chemicals derived from benzene 14

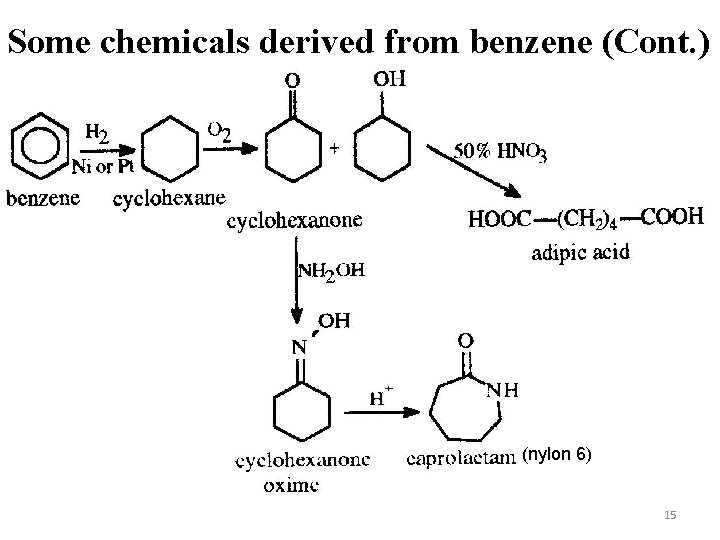
Some chemicals derived from benzene (Cont. ) (nylon 6) 15

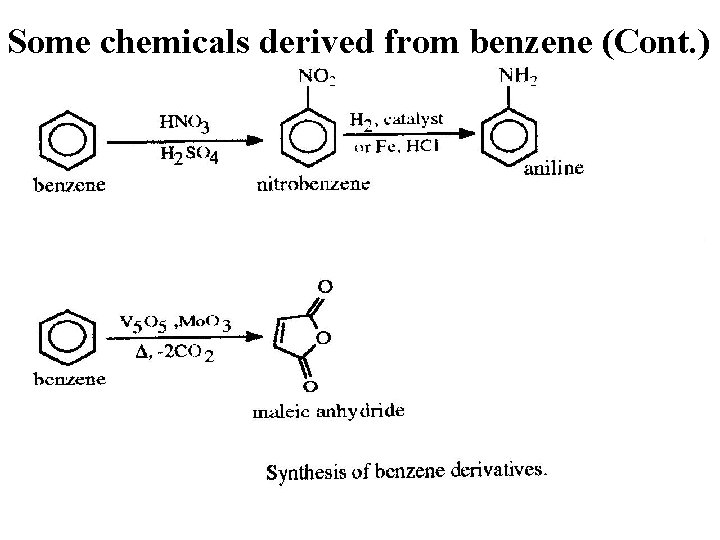
Some chemicals derived from benzene (Cont. ) P 215 -3 16

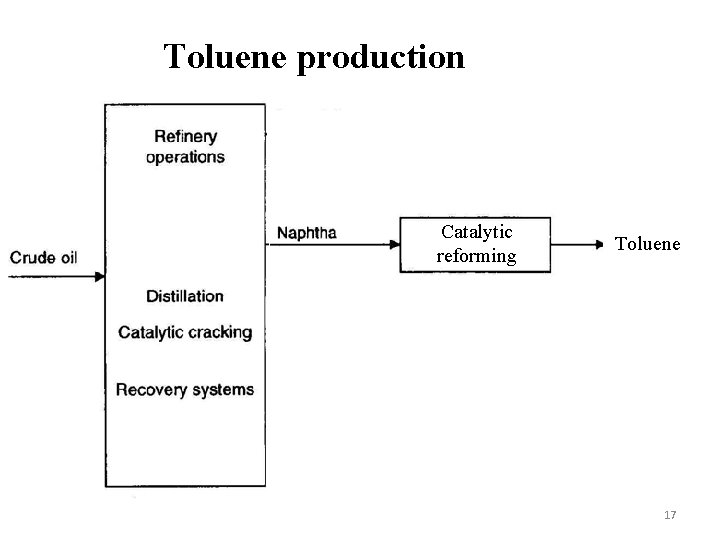
Toluene production Catalytic reforming Toluene 17

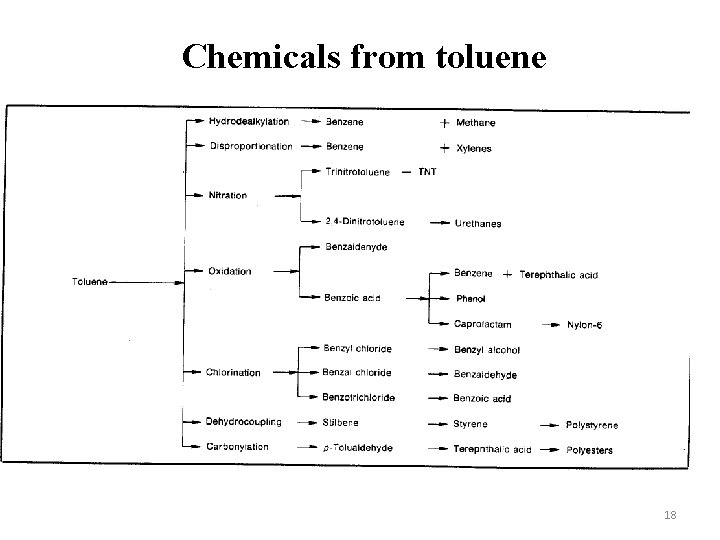
Chemicals from toluene 18

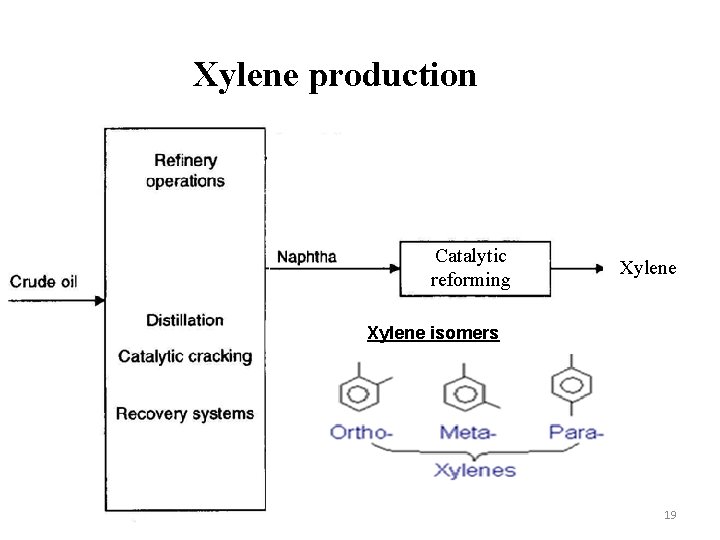
Xylene production Catalytic reforming Xylene isomers 19

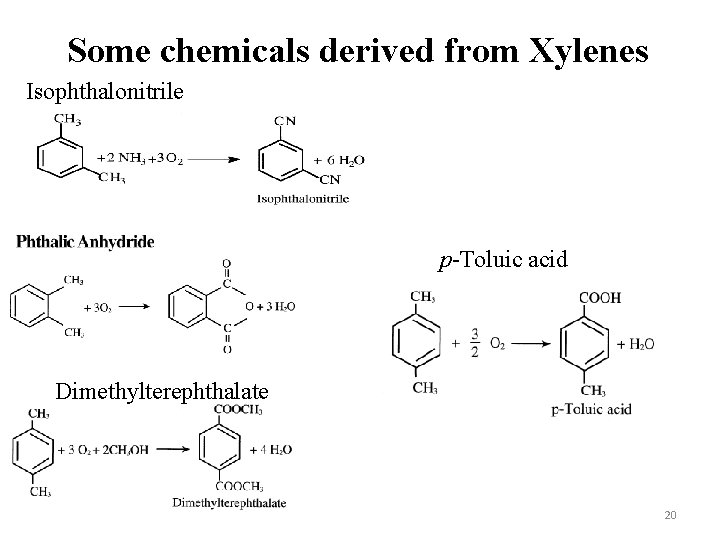
Some chemicals derived from Xylenes Isophthalonitrile p-Toluic acid Dimethylterephthalate 20

Major reactions in petrochemical production 1. Alkylation 2. Isomerization 3. Catalytic reforming 4. Disproportionation 5. Transalkylation 6. Selective oxidation 7. Dehydrogenation 21

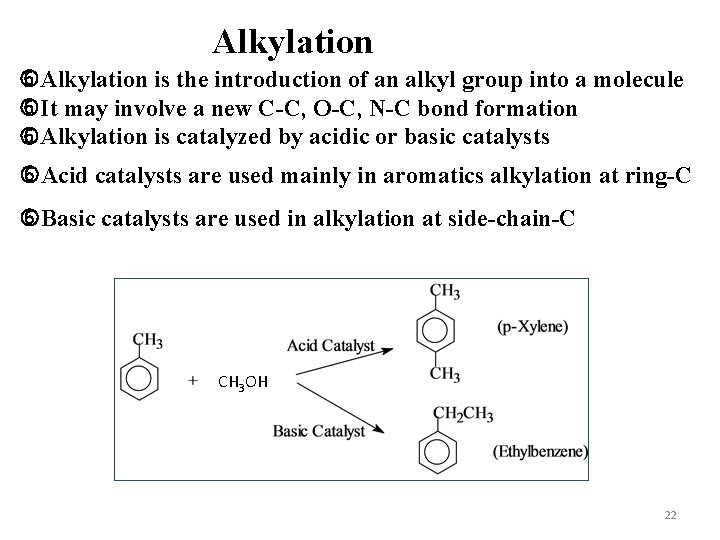
Alkylation is the introduction of an alkyl group into a molecule It may involve a new C-C, O-C, N-C bond formation Alkylation is catalyzed by acidic or basic catalysts Acid catalysts are used mainly in aromatics alkylation at ring-C Basic catalysts are used in alkylation at side-chain-C CH 3 OH 22

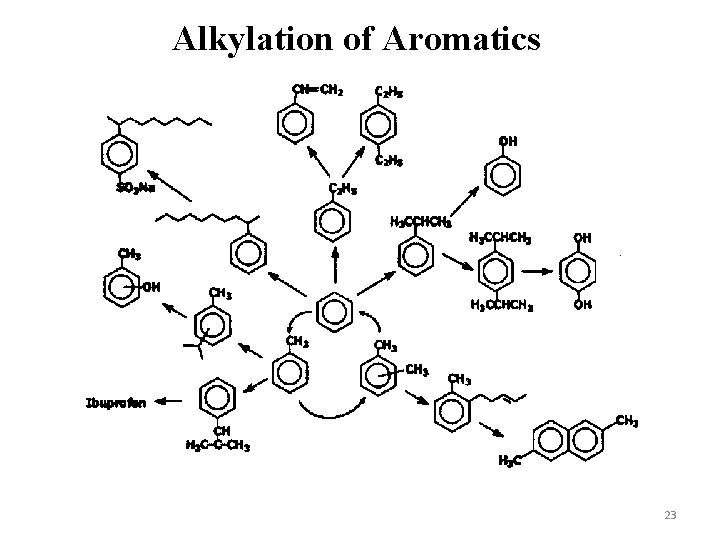
Alkylation of Aromatics 23

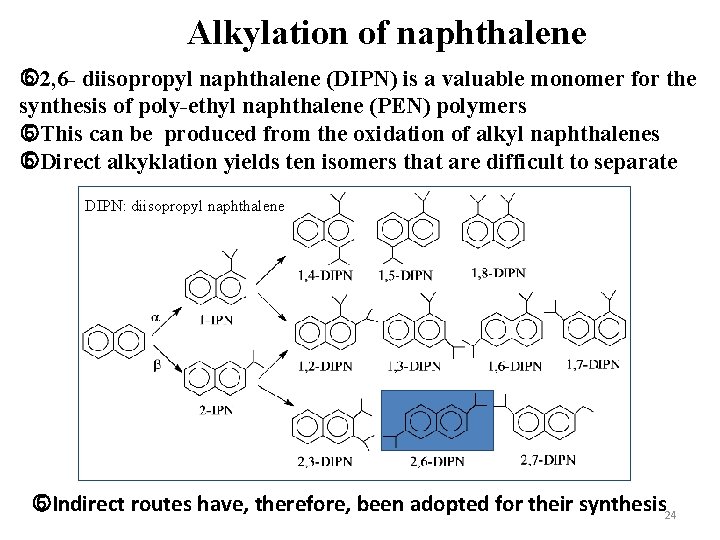
Alkylation of naphthalene 2, 6 - diisopropyl naphthalene (DIPN) is a valuable monomer for the synthesis of poly-ethyl naphthalene (PEN) polymers This can be produced from the oxidation of alkyl naphthalenes Direct alkyklation yields ten isomers that are difficult to separate DIPN: diisopropyl naphthalene Indirect routes have, therefore, been adopted for their synthesis 24

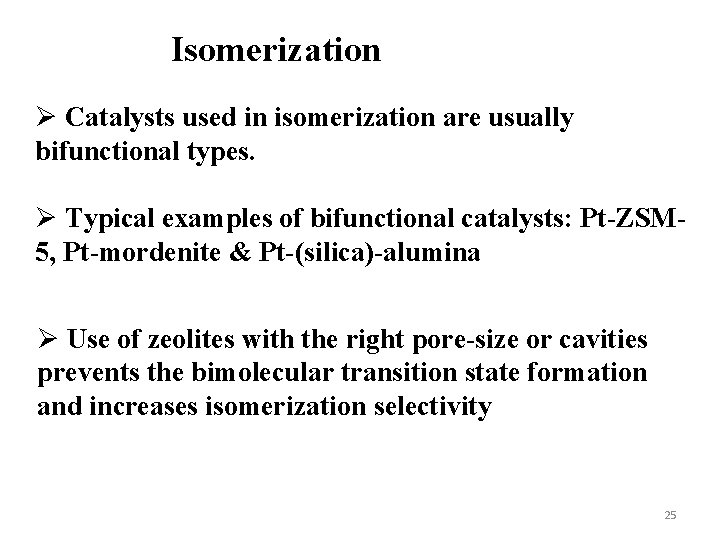
Isomerization Ø Catalysts used in isomerization are usually bifunctional types. Ø Typical examples of bifunctional catalysts: Pt-ZSM 5, Pt-mordenite & Pt-(silica)-alumina Ø Use of zeolites with the right pore-size or cavities prevents the bimolecular transition state formation and increases isomerization selectivity 25

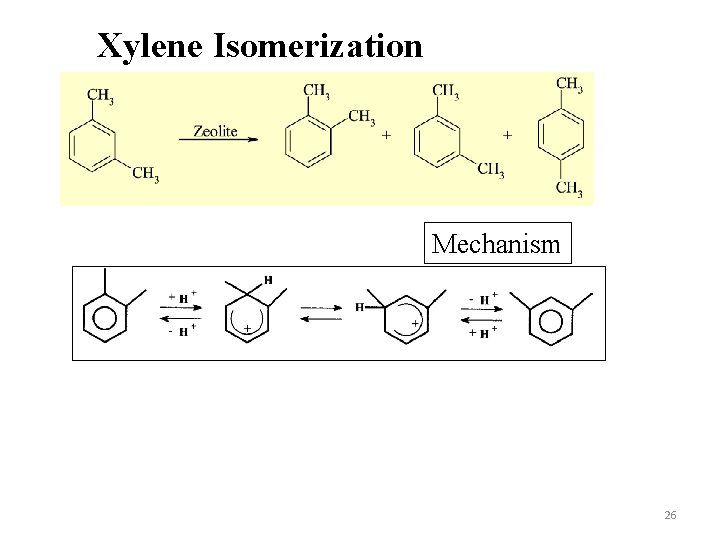
Xylene Isomerization Mechanism 26

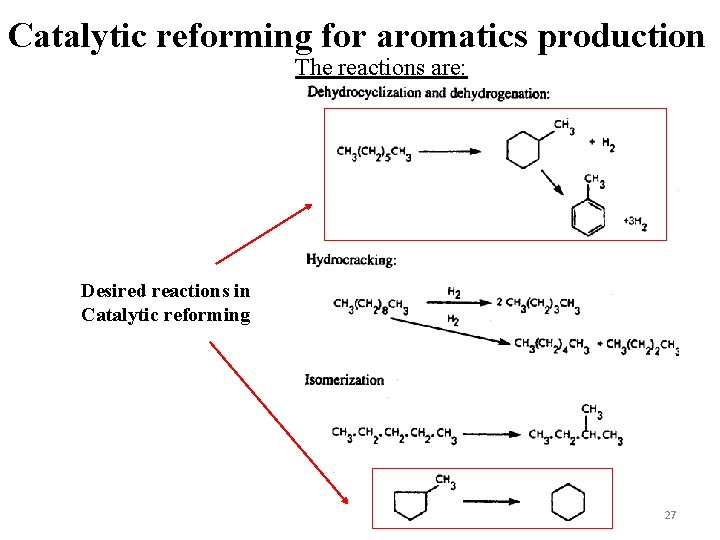
Catalytic reforming for aromatics production The reactions are: Desired reactions in Catalytic reforming 27

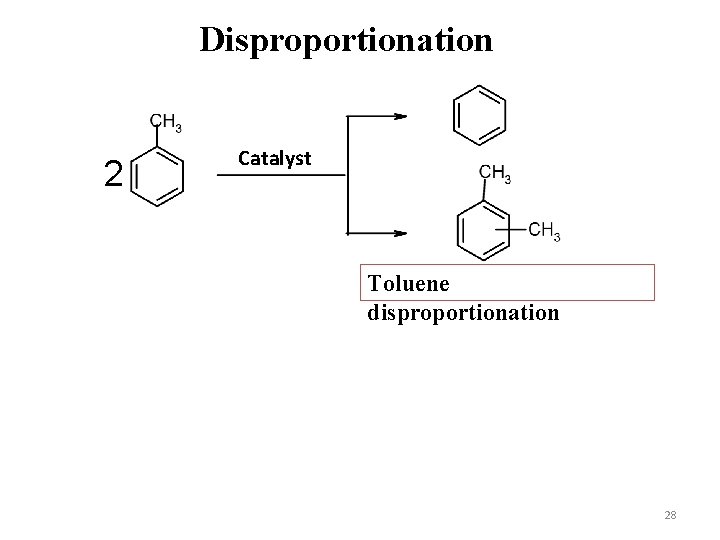
Disproportionation 2 Catalyst Toluene disproportionation 28

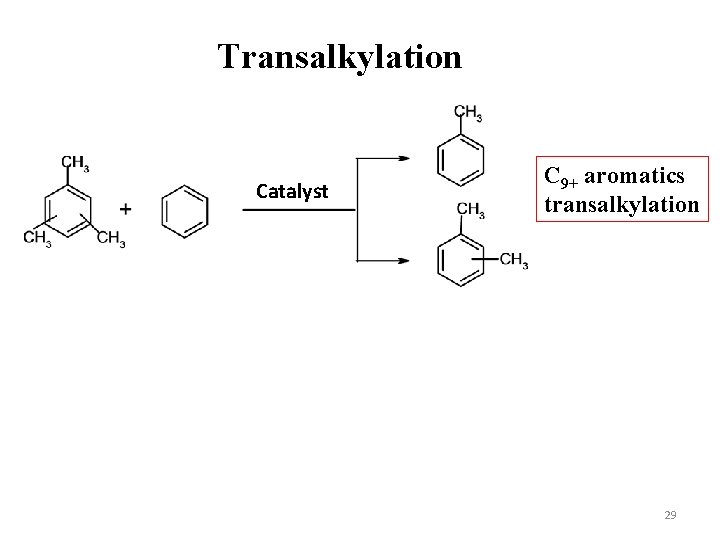
Transalkylation Catalyst C 9+ aromatics transalkylation 29

Selective oxidation reactions Oxidation of partially oxidized compounds is more favorable over oxidation of the initial reactants, resulting in low selectivity. Selective oxidation overcomes the above problem 30

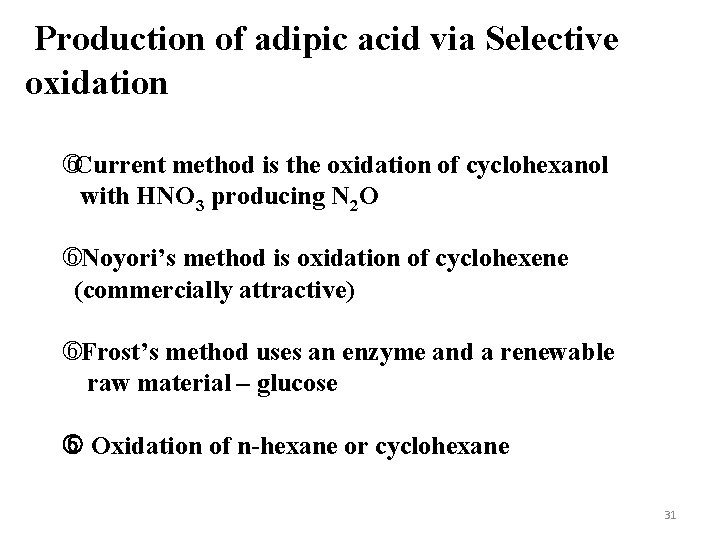
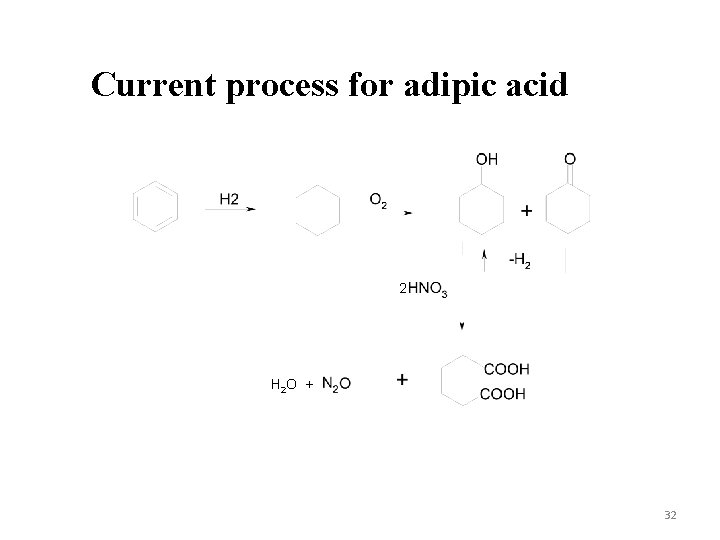
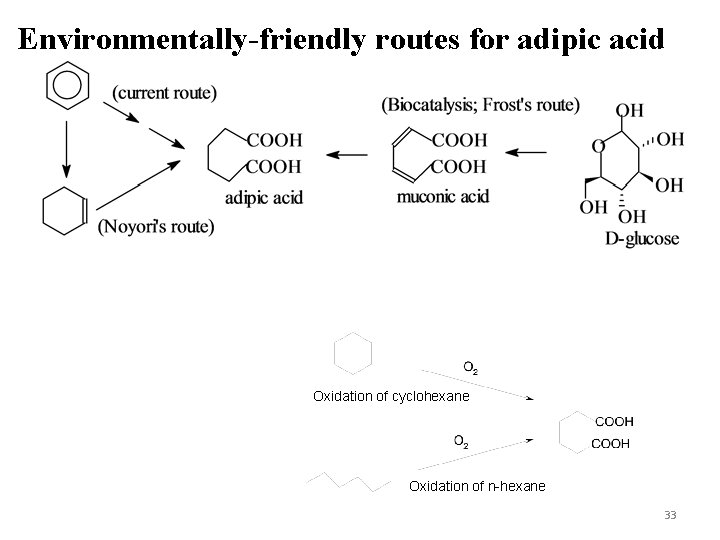
Production of adipic acid via Selective oxidation Current method is the oxidation of cyclohexanol with HNO 3 producing N 2 O Noyori’s method is oxidation of cyclohexene (commercially attractive) Frost’s method uses an enzyme and a renewable raw material – glucose Oxidation of n-hexane or cyclohexane 31

Current process for adipic acid 2 H 2 O + 32

Environmentally-friendly routes for adipic acid Oxidation of cyclohexane Oxidation of n-hexane 33

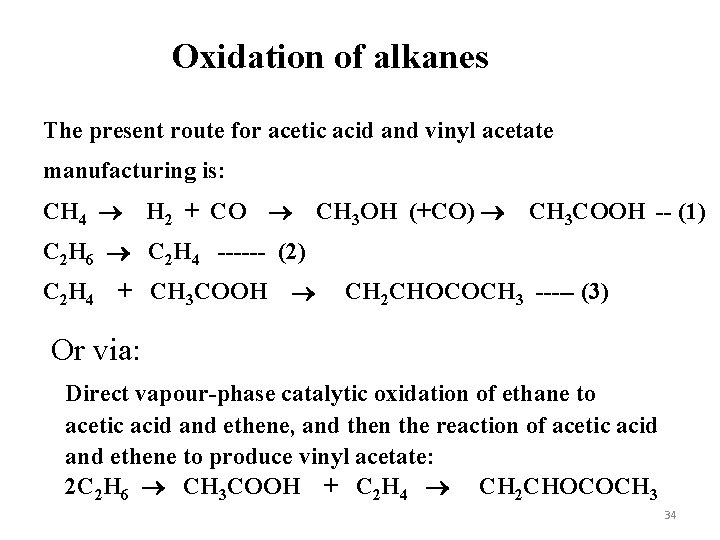
Oxidation of alkanes The present route for acetic acid and vinyl acetate manufacturing is: CH 4 H 2 + CO CH 3 OH (+CO) CH 3 COOH -- (1) C 2 H 6 C 2 H 4 ------ (2) C 2 H 4 + CH 3 COOH CH 2 CHOCOCH 3 ----- (3) Or via: Direct vapour-phase catalytic oxidation of ethane to acetic acid and ethene, and then the reaction of acetic acid and ethene to produce vinyl acetate: 2 C 2 H 6 CH 3 COOH + C 2 H 4 CH 2 CHOCOCH 3 34

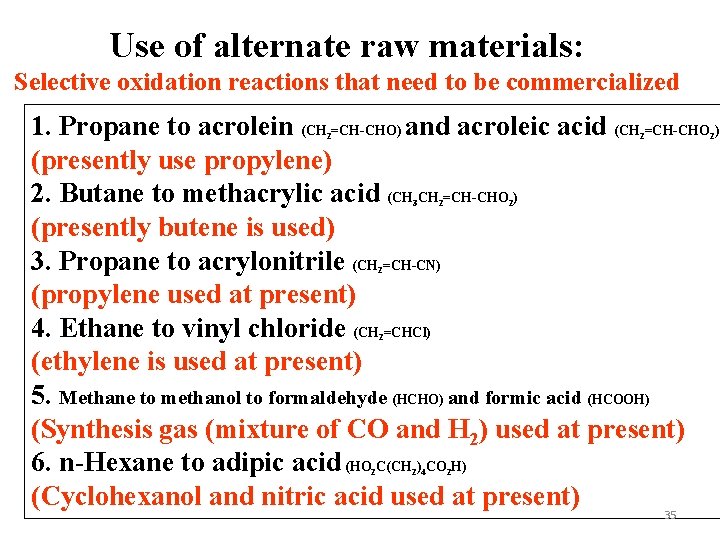
Use of alternate raw materials: Selective oxidation reactions that need to be commercialized 1. Propane to acrolein (CH =CH-CHO) and acroleic acid (CH =CH-CHO ) (presently use propylene) 2. Butane to methacrylic acid (CH CH =CH-CHO ) (presently butene is used) 3. Propane to acrylonitrile (CH =CH-CN) (propylene used at present) 4. Ethane to vinyl chloride (CH =CHCl) (ethylene is used at present) 5. Methane to methanol to formaldehyde (HCHO) and formic acid (HCOOH) (Synthesis gas (mixture of CO and H 2) used at present) 6. n-Hexane to adipic acid (HO C(CH ) CO H) (Cyclohexanol and nitric acid used at present) 2 2 3 2 2 2 2 4 2 35

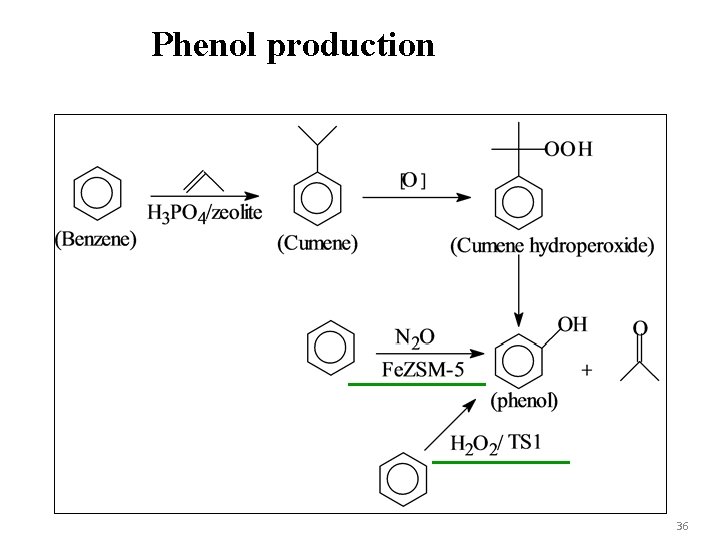
Phenol production 36

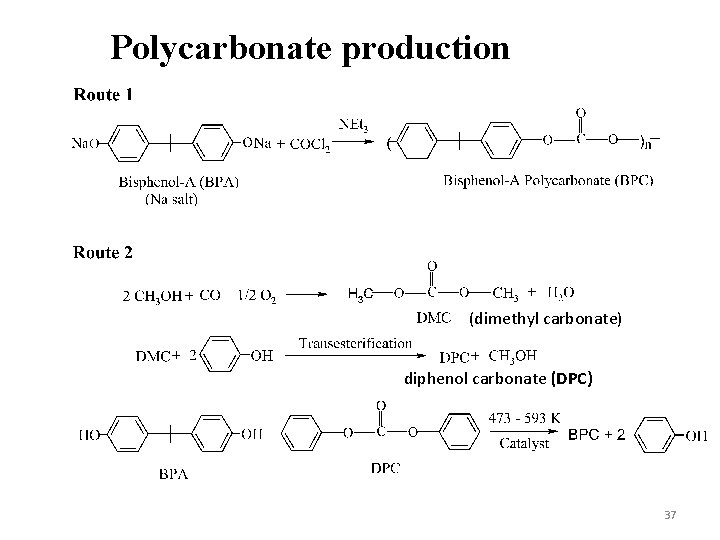
Polycarbonate production (dimethyl carbonate) diphenol carbonate (DPC) 37

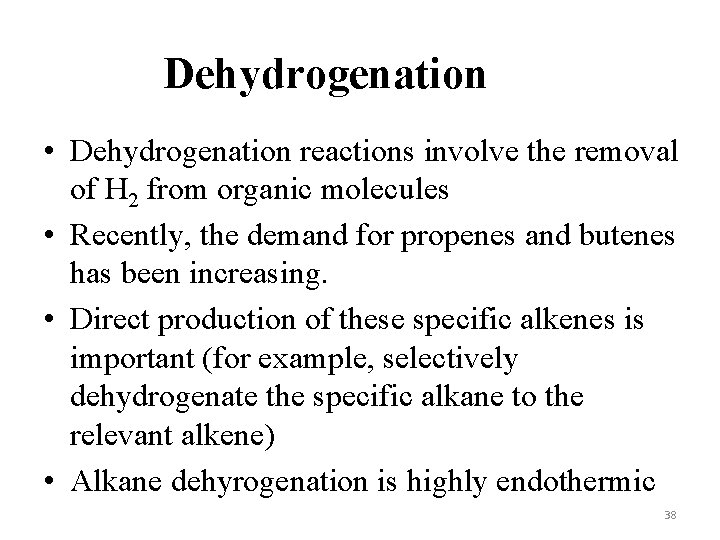
Dehydrogenation • Dehydrogenation reactions involve the removal of H 2 from organic molecules • Recently, the demand for propenes and butenes has been increasing. • Direct production of these specific alkenes is important (for example, selectively dehydrogenate the specific alkane to the relevant alkene) • Alkane dehyrogenation is highly endothermic 38

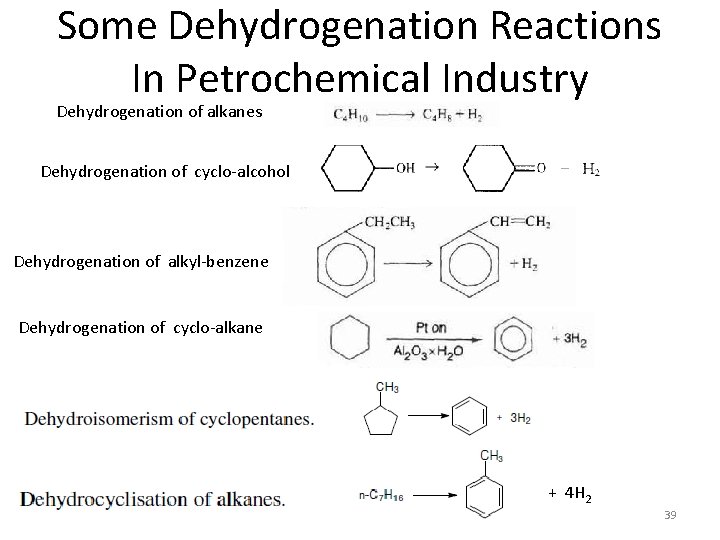
Some Dehydrogenation Reactions In Petrochemical Industry Dehydrogenation of alkanes Dehydrogenation of cyclo-alcohol Dehydrogenation of alkyl-benzene Dehydrogenation of cyclo-alkane + 4 H 2 39

End of Petrochemicals 40
 Mikael ferm
Mikael ferm Petrochemical industry statistics
Petrochemical industry statistics Cpq petrochemicals industry
Cpq petrochemicals industry Strengthscape
Strengthscape Petrochemical demand
Petrochemical demand What does petrochemical mean
What does petrochemical mean Petrochemicals
Petrochemicals Egyptian petrochemicals holding company
Egyptian petrochemicals holding company Redogör för vad psykologi är
Redogör för vad psykologi är Särskild löneskatt för pensionskostnader
Särskild löneskatt för pensionskostnader Borra hål för knoppar
Borra hål för knoppar Bris för vuxna
Bris för vuxna Mat för idrottare
Mat för idrottare Lek med former i förskolan
Lek med former i förskolan Svenskt ramverk för digital samverkan
Svenskt ramverk för digital samverkan Ledarskapsteorier
Ledarskapsteorier Datorkunskap för nybörjare
Datorkunskap för nybörjare Vad kallas den mantel som bars av kvinnor i antikens rom
Vad kallas den mantel som bars av kvinnor i antikens rom Rita perspektiv
Rita perspektiv Ekologiskt fotavtryck
Ekologiskt fotavtryck Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Sju principer för tillitsbaserad styrning
Sju principer för tillitsbaserad styrning Plats för toran ark
Plats för toran ark Claes martinsson
Claes martinsson Olika rim dikter
Olika rim dikter Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Tidbok för yrkesförare
Tidbok för yrkesförare Handledning reflektionsmodellen
Handledning reflektionsmodellen Stål för stötfångarsystem
Stål för stötfångarsystem Orubbliga rättigheter
Orubbliga rättigheter Bamse för de yngsta
Bamse för de yngsta Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Texter för hinduer tantra
Texter för hinduer tantra Fspos
Fspos Jag har nigit för nymånens skära
Jag har nigit för nymånens skära Skivepiteldysplasi
Skivepiteldysplasi Boverket ka
Boverket ka Strategi för svensk viltförvaltning
Strategi för svensk viltförvaltning Romarriket tidslinje
Romarriket tidslinje Verksamhetsanalys exempel
Verksamhetsanalys exempel Tack för att ni har lyssnat
Tack för att ni har lyssnat