Periodic Trends Section 6 3 Periodic Law The







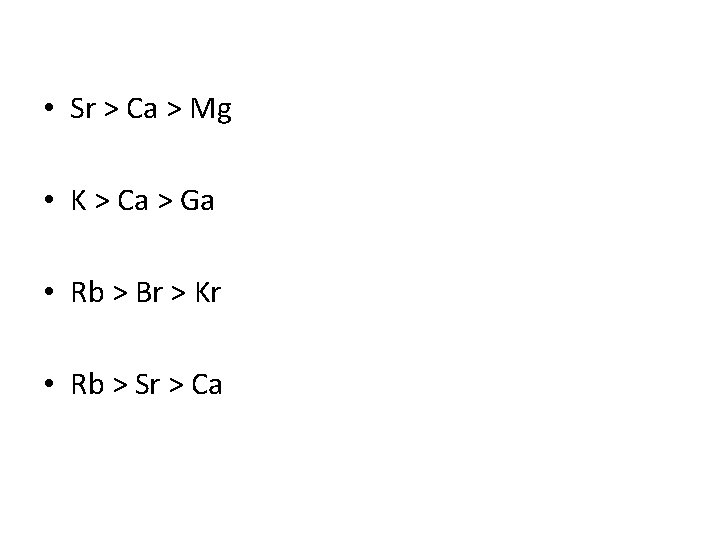









- Slides: 21

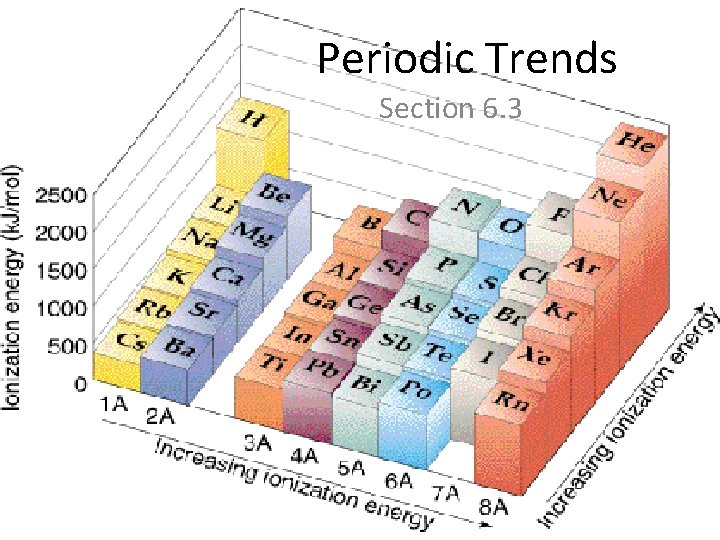
Periodic Trends Section 6. 3

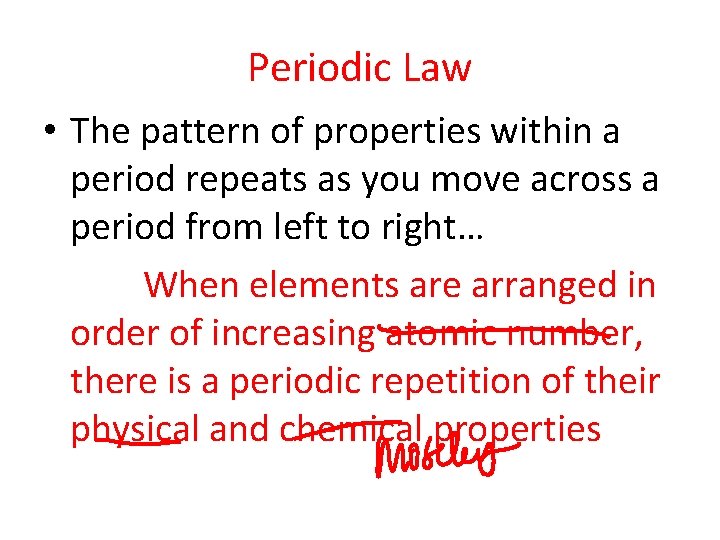
Periodic Law • The pattern of properties within a period repeats as you move across a period from left to right… When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties

Periodic Trends • Trend – a predictable change • Our focus will be on the main block elements • How do electron configurations help us explain many of the trends in properties observed?

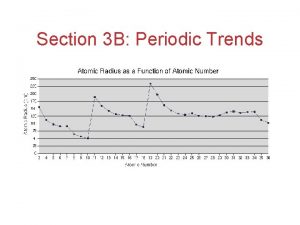
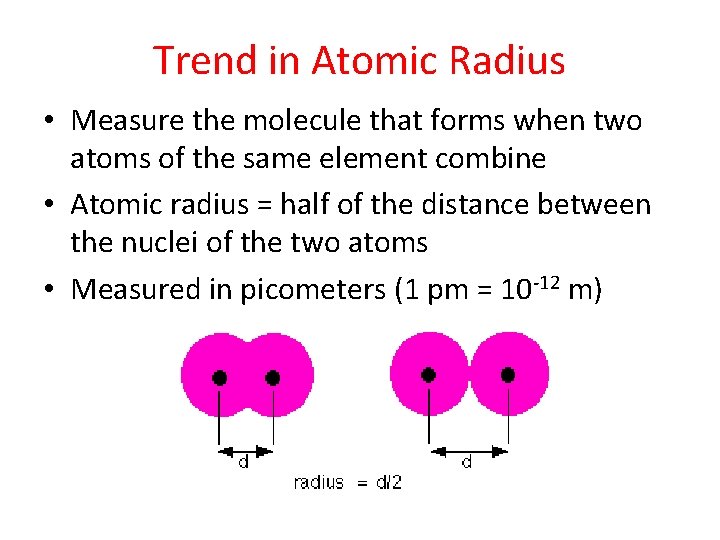
Trend in Atomic Radius • Measure the molecule that forms when two atoms of the same element combine • Atomic radius = half of the distance between the nuclei of the two atoms • Measured in picometers (1 pm = 10 -12 m)



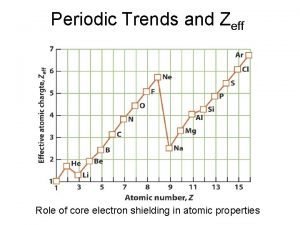
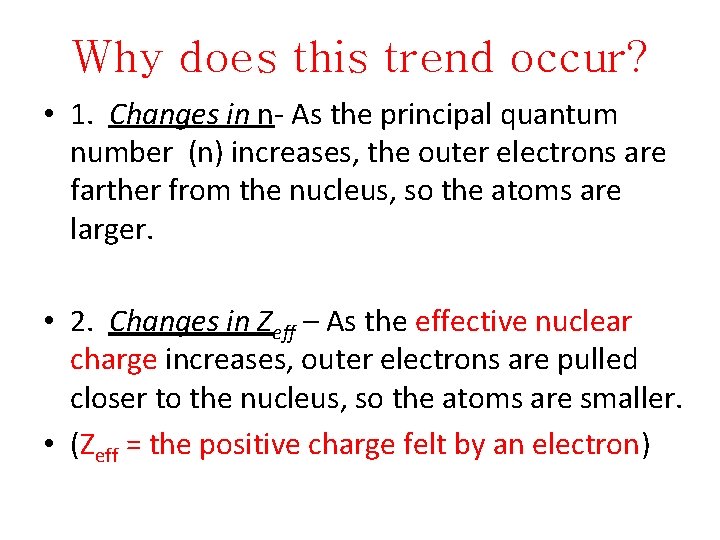
Why does this trend occur? • 1. Changes in n- As the principal quantum number (n) increases, the outer electrons are farther from the nucleus, so the atoms are larger. • 2. Changes in Zeff – As the effective nuclear charge increases, outer electrons are pulled closer to the nucleus, so the atoms are smaller. • (Zeff = the positive charge felt by an electron)

1. Down a group, n dominates • Elements have one more energy level of core electrons • These SHIELD the outer electrons • Atomic radius generally INCREASES in a group from top to bottom

2. Across a period, Zeff dominates • Moving across a period, electrons are added to the same outer level • Shielding by inner electrons does not change • Zeff increases and outer electrons are pulled closer • Atomic radius generally DECREASES in a period from left to right


Try the following… • Put in order of decreasing atomic size • Ca, Mg, Sr • K, Ga, Ca • Br, Rb, Kr • Sr, Ca, Rb
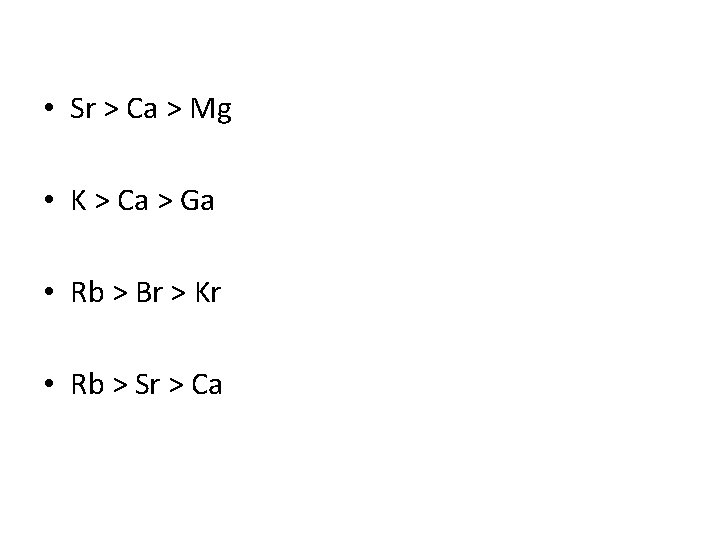
• Sr > Ca > Mg • K > Ca > Ga • Rb > Br > Kr • Rb > Sr > Ca

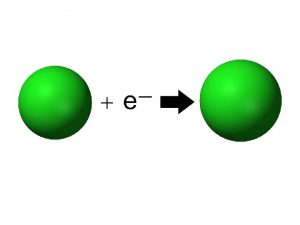
Ions • Atom or group of atoms that has a positive or negative charge • Form when electrons are transferred between atoms • Metals tend to lose electrons, forming cations • Nonmetals gain electrons, forming anions


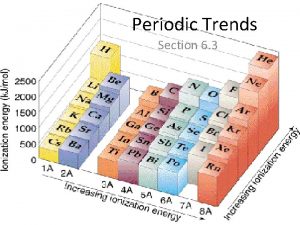
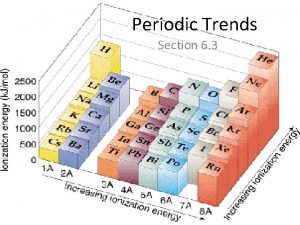
Trends in Ionization Energy • Energy needed to remove an electron from an atom • Measured when element is in its gaseous state • Energy needed to remove the first electron from an atom is the FIRST IONIZATION ENERGY • Produces cation with 1+ charge

• First ionization energy tends to decrease from top to bottom in a group and increases from left to right across a period


Why does this trend occur? • As atomic size increases down a group, Zeff has a smaller effect on the electrons in highest level • Shielding Effect • Less energy is needed to remove electron from the energy level making first ionization energy lower

Across a period… • Zeff increases and shielding effect remains constant • Increase in the attraction the nucleus has for an electron • More energy is needed to remove an electron from the atom and first ionization energy is higher

Try these… • Put the following in order of decreasing IE 1 • Kr, He, Ar • Sb, Te, Sn • K, Ca, Rb • I, Xe, Cs

• He > Ar > Kr • Te > Sb > Sn • Ca > K > Rb • Xe > I > Cs
 Trends of the periodic table
Trends of the periodic table Elements in period 2
Elements in period 2 Electron affinity worksheet
Electron affinity worksheet Chapter 6 the periodic table
Chapter 6 the periodic table Chapter 6 periodic table
Chapter 6 periodic table Chapter 5 periodic law
Chapter 5 periodic law Electronegativity vs ionization energy
Electronegativity vs ionization energy Periodic trends
Periodic trends Ultimate periodic table cheat sheet
Ultimate periodic table cheat sheet Oxygen periodic trends
Oxygen periodic trends Graphing periodic trends
Graphing periodic trends Oxidation trends periodic table
Oxidation trends periodic table Periodic trends electronegativity
Periodic trends electronegativity Periodic trend of zeff
Periodic trend of zeff Periodic trends activity worksheet
Periodic trends activity worksheet Periodic trends practice questions
Periodic trends practice questions Periodic trends reactivity
Periodic trends reactivity Summary of periodic trends
Summary of periodic trends Periodic table practice problems
Periodic table practice problems Highest electron affinity
Highest electron affinity Increasing atomic size
Increasing atomic size Is sulfer a cation or anion
Is sulfer a cation or anion