Organic Compounds Essential Questions What is Organic What










- Slides: 25

Organic Compounds Essential Questions: What is “Organic? ” What are the 4 major Organic Compounds? How are they made? What are they used for?

What does “Organic” Mean? In Biology, organic means “relating to organisms. ” NOT food grown without the use of pesticides, antibiotics, or other industrial chemicals. What do all organic compounds contain? CARBON All organic molecules contain covalently bonded Carbon.

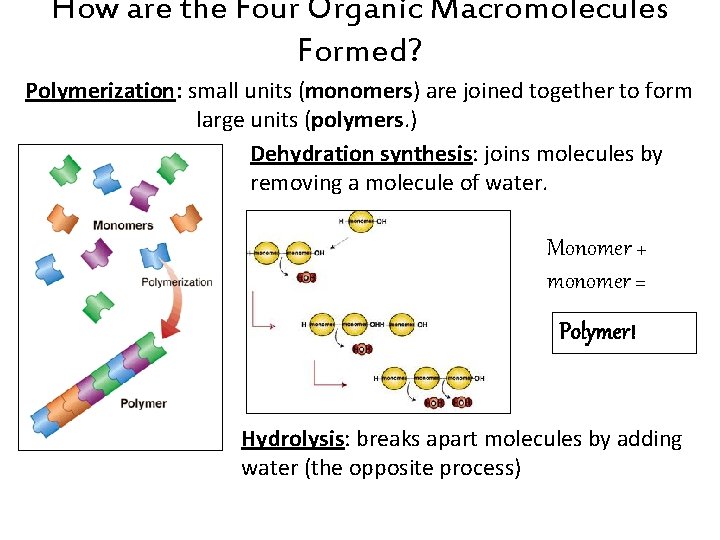
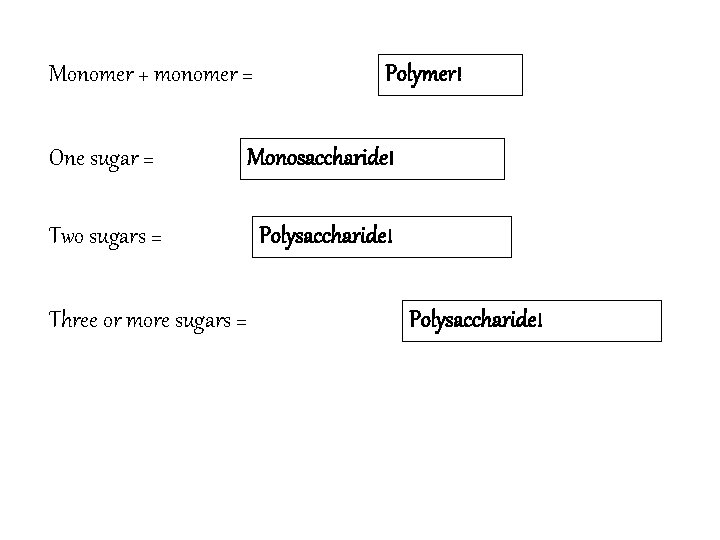
How are the Four Organic Macromolecules Formed? Polymerization: small units (monomers) are joined together to form large units (polymers. ) Dehydration synthesis: joins molecules by removing a molecule of water. Monomer + monomer = Polymer! Hydrolysis: breaks apart molecules by adding water (the opposite process)

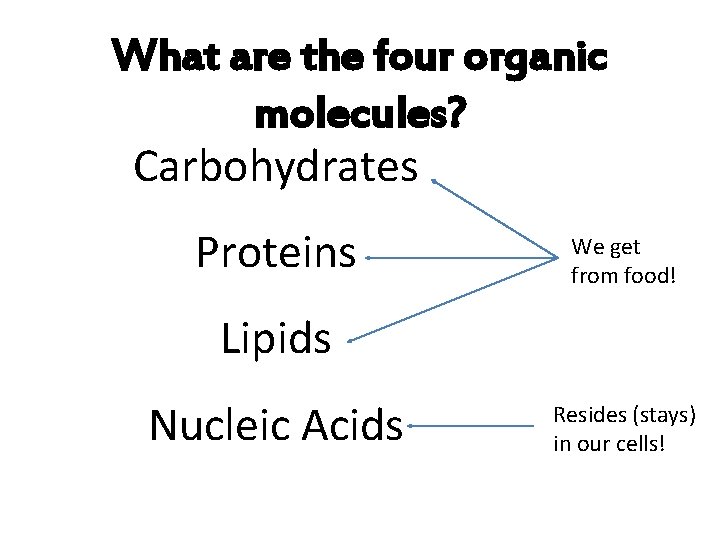
What are the four organic molecules? Carbohydrates Proteins We get from food! Lipids Nucleic Acids Resides (stays) in our cells!

What is in a cheeseburger? Nutrition Facts (Big ‘N Tasty at Mc. Donalds): Total Fat: 29 grams Saturated Fat: 9 grams Carbohydrates: 41 grams Protein: 24 grams

Carbohydrates Which part of the cheeseburger has the most carbohydrates?

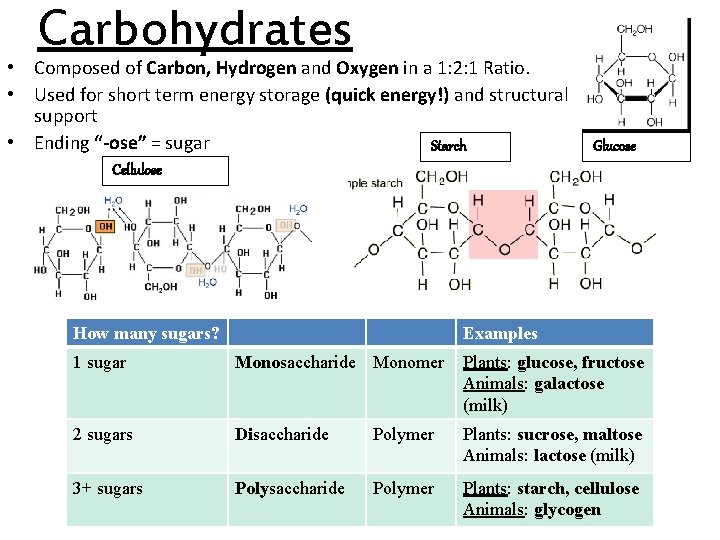
Carbohydrates • Composed of Carbon, Hydrogen and Oxygen in a 1: 2: 1 Ratio. • Used for short term energy storage (quick energy!) and structural support • Ending “-ose” = sugar Starch Glucose Cellulose How many sugars? Examples 1 sugar Monosaccharide Monomer Plants: glucose, fructose Animals: galactose (milk) 2 sugars Disaccharide Polymer Plants: sucrose, maltose Animals: lactose (milk) 3+ sugars Polysaccharide Polymer Plants: starch, cellulose Animals: glycogen

Monomer + monomer = Polymer! One sugar = Monosaccharide! Two sugars = Polysaccharide! Three or more sugars = Polysaccharide!

Why Carbohydrates? • Many animals store extra sugar as glycogen. – Glycogen stored in your muscles supplies energy for movement. – Glycogen stored in your liver is released when the glucose (sugar) in your blood runs low. • Recall: What is this an example of? – Homeostasis! • Plants store excess sugar as starch. • Plants also make cellulose, a strong, rigid fiber used for support. Starch, cellulose and glycogen are all… Polysaccharides!

What other part of the Big N’ Tasty is composed of carbohydrates? Cellulose!

Which part of the cheeseburger is the best source of protein?

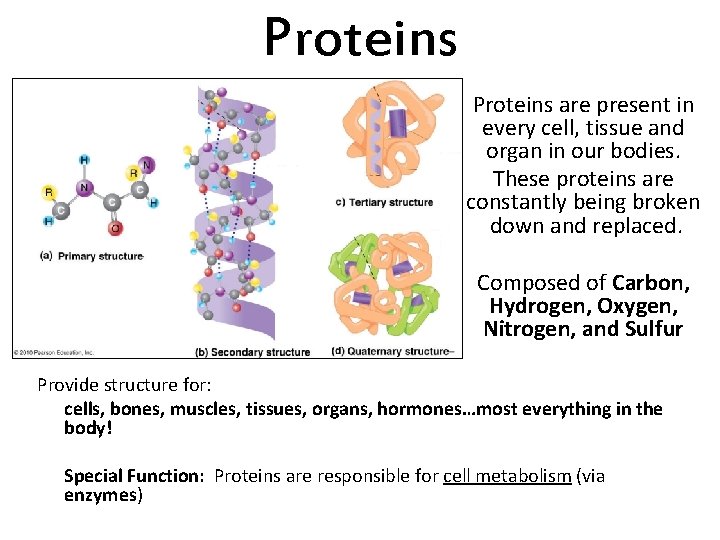
Proteins are present in every cell, tissue and organ in our bodies. These proteins are constantly being broken down and replaced. Composed of Carbon, Hydrogen, Oxygen, Nitrogen, and Sulfur Provide structure for: cells, bones, muscles, tissues, organs, hormones…most everything in the body! Special Function: Proteins are responsible for cell metabolism (via enzymes)

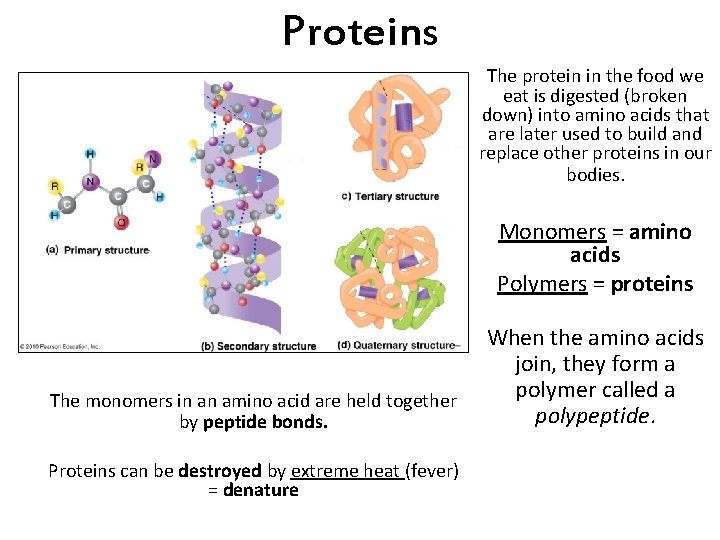
Proteins The protein in the food we eat is digested (broken down) into amino acids that are later used to build and replace other proteins in our bodies. Monomers = amino acids Polymers = proteins The monomers in an amino acid are held together by peptide bonds. Proteins can be destroyed by extreme heat (fever) = denature When the amino acids join, they form a polymer called a polypeptide.

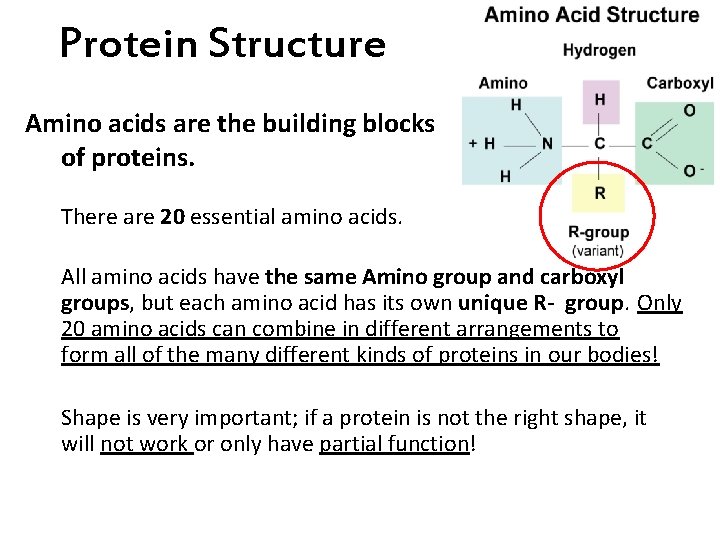
Protein Structure Amino acids are the building blocks of proteins. There are 20 essential amino acids. All amino acids have the same Amino group and carboxyl groups, but each amino acid has its own unique R- group. Only 20 amino acids can combine in different arrangements to form all of the many different kinds of proteins in our bodies! Shape is very important; if a protein is not the right shape, it will not work or only have partial function!

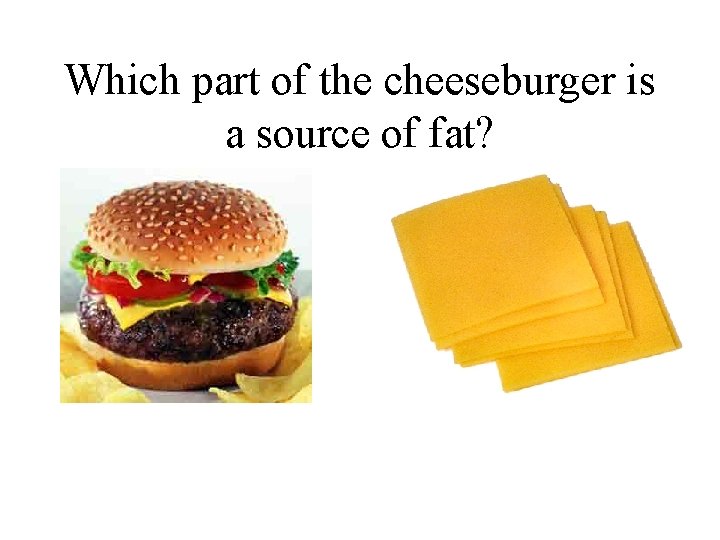
Which part of the cheeseburger is a source of fat?

What is fat? • Fats are a type of lipid. • Lipids are hydrophobic (water-fearing) organic molecule including fats, oils, waxes, phospholipids and steroids.

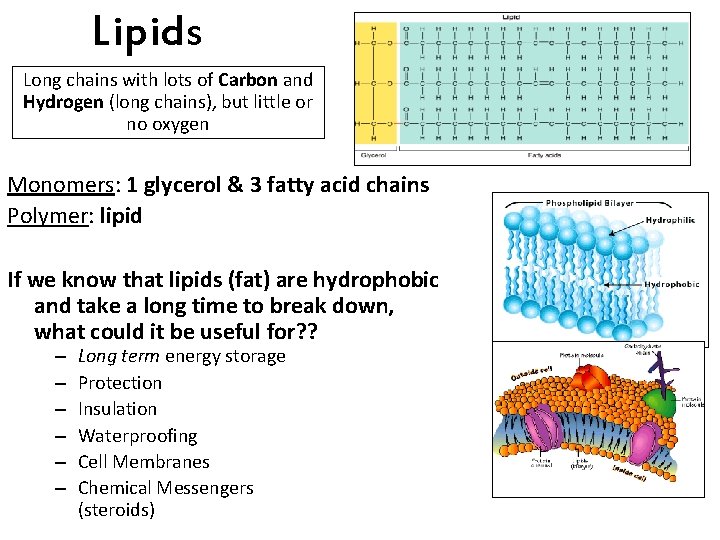
Lipids Long chains with lots of Carbon and Hydrogen (long chains), but little or no oxygen Monomers: 1 glycerol & 3 fatty acid chains Polymer: lipid If we know that lipids (fat) are hydrophobic and take a long time to break down, what could it be useful for? ? – – – Long term energy storage Protection Insulation Waterproofing Cell Membranes Chemical Messengers (steroids)

Lipids come in two flavors… Saturated: Single Bonds • • Unsaturated: Double Bonds Animal Fats Harder to digest Solids at room temperature Holds as many Hydrogen atoms as possible • • Vegetable Oils Easier to digest Liquids at room Temperature Does not hold as many hydrogen atoms as possible Your Turn! Make a quick hypothesis to why Unsaturated Fats are easier to break down (thus healthier for you) than Saturated Fats! It is easier for your body to break double bonds than single bonds due to the number of electrons. Aka, it’s easier to steal 1 electron from Carbon when it is sharing two versus just that one! (Like borrowing money!)

Common Misconceptions: Lipids – Good & Bad Cholesterol You’ll be amazed at what you find online about the different types of cholesterol! You should check it out!

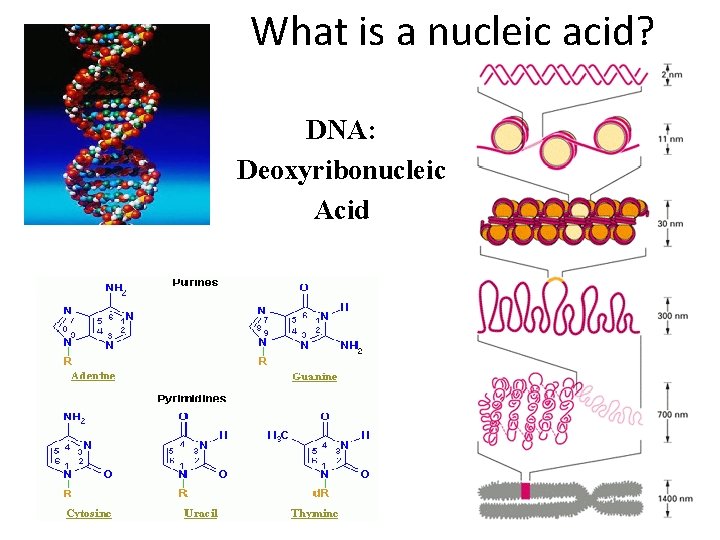
What is a nucleic acid? DNA: Deoxyribonucleic Acid

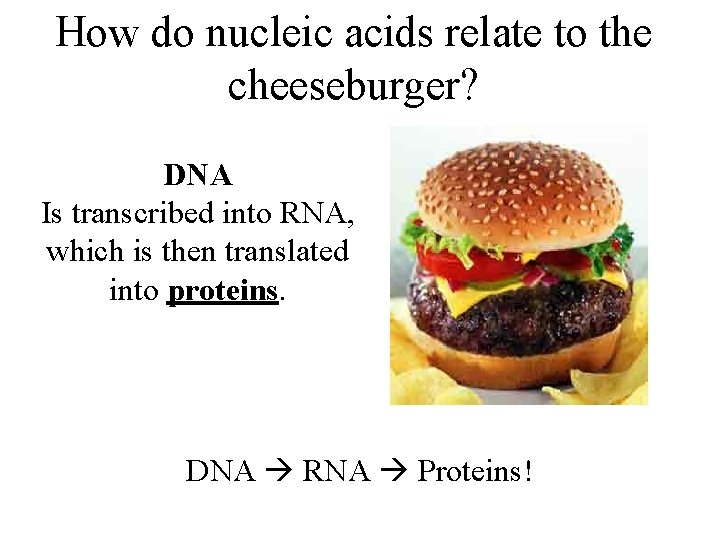
How do nucleic acids relate to the cheeseburger? DNA Is transcribed into RNA, which is then translated into proteins. DNA RNA Proteins!

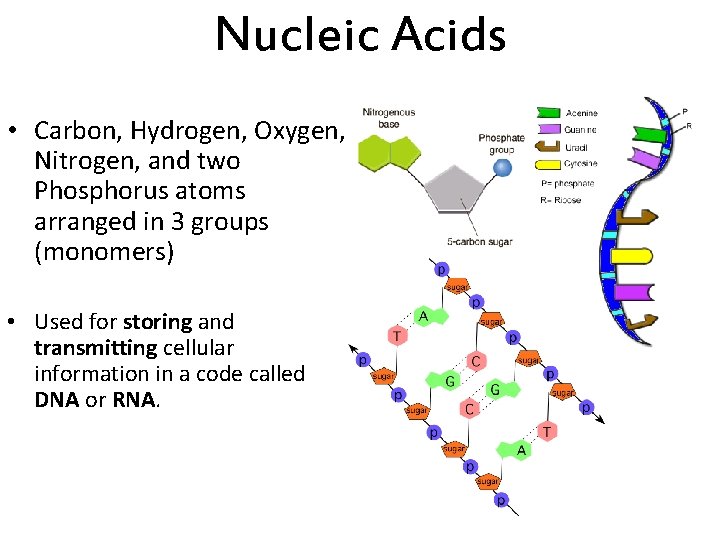
Nucleic Acids • Carbon, Hydrogen, Oxygen, Nitrogen, and two Phosphorus atoms arranged in 3 groups (monomers) • Used for storing and transmitting cellular information in a code called DNA or RNA.

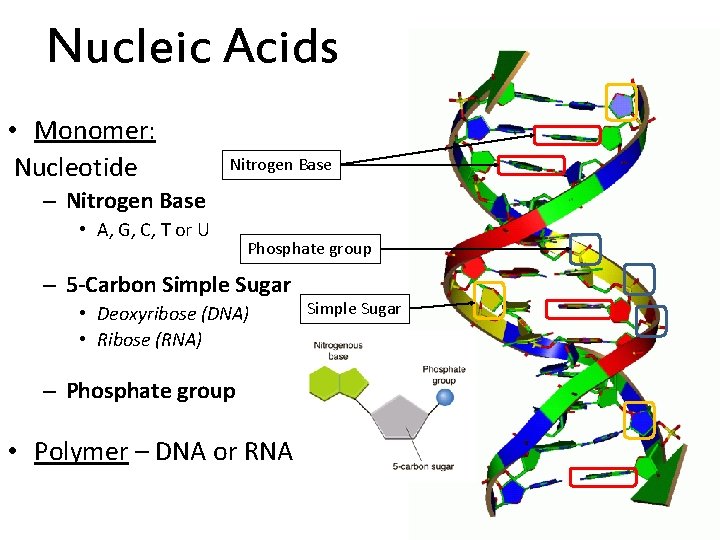
Nucleic Acids • Monomer: Nucleotide Nitrogen Base – Nitrogen Base • A, G, C, T or U Phosphate group – 5 -Carbon Simple Sugar • Deoxyribose (DNA) • Ribose (RNA) – Phosphate group • Polymer – DNA or RNA Simple Sugar

Organic molecules are the building blocks of life They are broken down into monomers, then rebuilt into polymers, then broken down again, then rebuilt again! And so life goes on…

Your turn! What kind of macromolecules did you/will you eat today?
 Ionic covalent and metallic venn diagram
Ionic covalent and metallic venn diagram Essential non essential fatty acids
Essential non essential fatty acids Are vitamins tasteless
Are vitamins tasteless Are vitamins organic compounds
Are vitamins organic compounds The four major groups of organic compounds are
The four major groups of organic compounds are Decomposition of organic compounds
Decomposition of organic compounds Charring test of organic and inorganic compounds
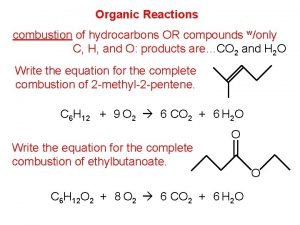
Charring test of organic and inorganic compounds Combustion reaction
Combustion reaction Biomolecule
Biomolecule All organic compounds must contain the element
All organic compounds must contain the element Organic compounds must contain
Organic compounds must contain Chemistry nomenclature
Chemistry nomenclature What is organic chemistry
What is organic chemistry Whats the difference between organic and inorganic
Whats the difference between organic and inorganic Purification and characterization of organic compounds
Purification and characterization of organic compounds Organic compounds such as proteins and starches are too
Organic compounds such as proteins and starches are too Organic vs inorganic compounds
Organic vs inorganic compounds What is the classification of organic compounds
What is the classification of organic compounds Carbon compound
Carbon compound Principle of simple distillation
Principle of simple distillation Reactions of organic compounds
Reactions of organic compounds Organic compounds grade 10 life science
Organic compounds grade 10 life science Organic halogen compounds
Organic halogen compounds Hemoragea
Hemoragea Metabolic changes of drugs and related organic compounds
Metabolic changes of drugs and related organic compounds All organic compounds contain carbon and ________.
All organic compounds contain carbon and ________.