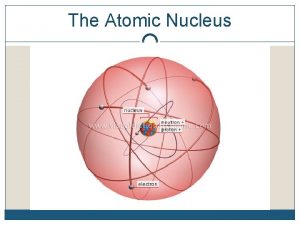
Nuclear Chemistry The weird world of the nucleus
















- Slides: 32

Nuclear Chemistry The weird world of the nucleus

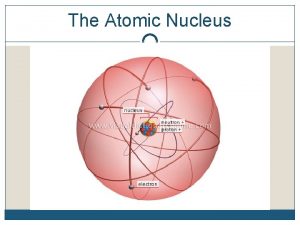
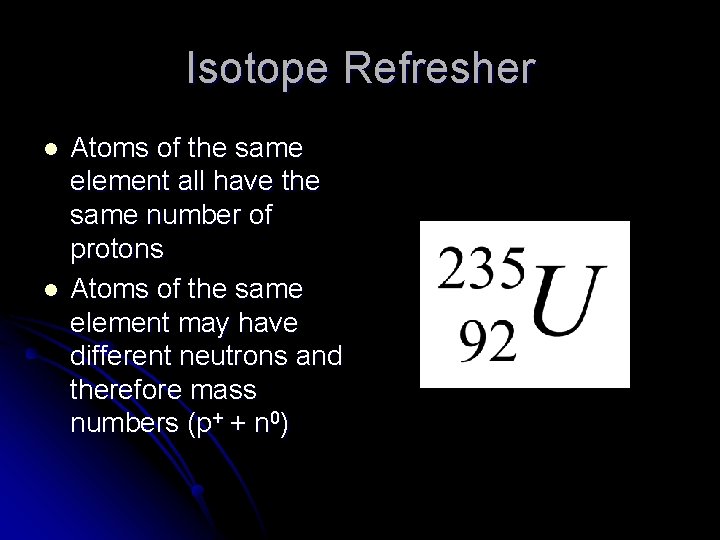
Isotope Refresher l l Atoms of the same element all have the same number of protons Atoms of the same element may have different neutrons and therefore mass numbers (p+ + n 0)

Nuclear Instability Not all combinations of protons and neutrons are created equal l Some are more unstable than others. l If they are unstable they will do one of the following: l l Radioactive decay l Nuclear fission l Nuclear fusion

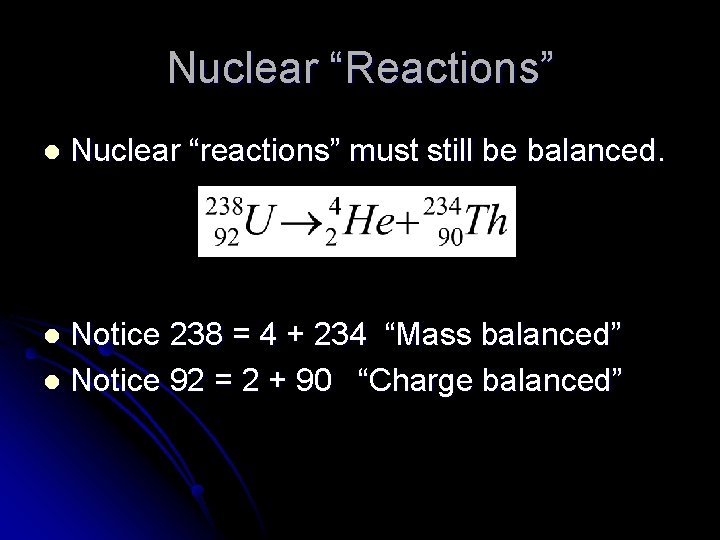
Nuclear “Reactions” l Nuclear “reactions” must still be balanced. Notice 238 = 4 + 234 “Mass balanced” l Notice 92 = 2 + 90 “Charge balanced” l

Radioactive Decay

Radioactive Decay l Radioactive decay – the nucleus of an atom undergoes a change so that it is no longer the same element l Decay is a totally random event. Nothing has an effect when an atom decays Two Main Types of Radioactive Decay 1. Alpha decay 2. Beta decay

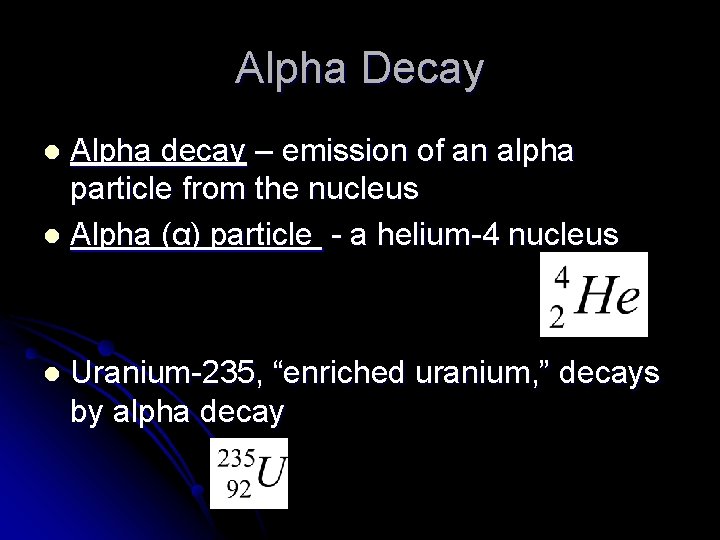
Alpha Decay Alpha decay – emission of an alpha particle from the nucleus l Alpha (α) particle - a helium-4 nucleus l l Uranium-235, “enriched uranium, ” decays by alpha decay

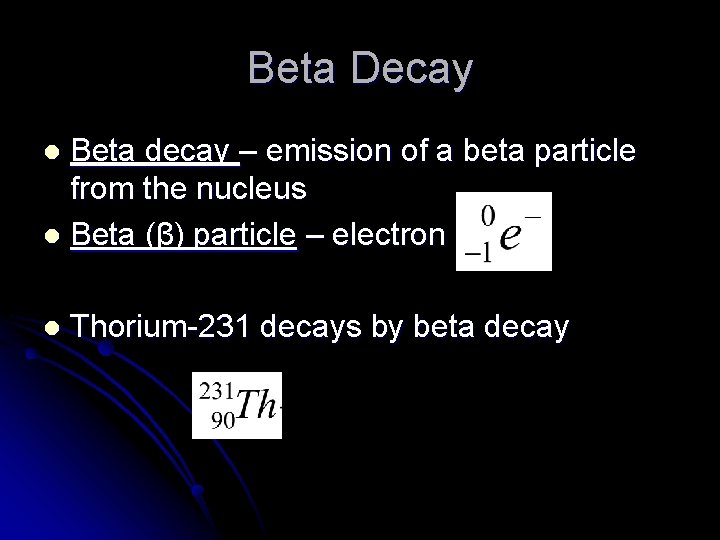
Beta Decay Beta decay – emission of a beta particle from the nucleus l Beta (β) particle – electron l l Thorium-231 decays by beta decay

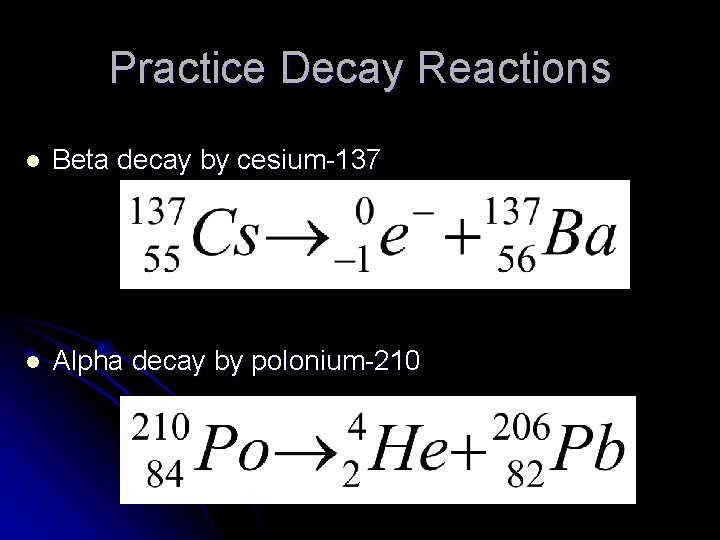
Practice Decay Reactions l Beta decay by cesium-137 l Alpha decay by polonium-210

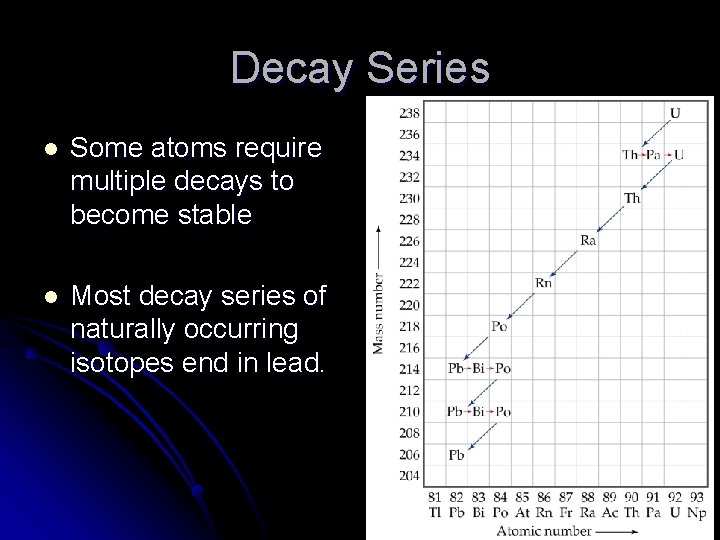
Decay Series l Some atoms require multiple decays to become stable l Most decay series of naturally occurring isotopes end in lead.


Half Life

Half Life l Half life – the amount of time it takes for ½ of a radioactive isotope to decay into something else. l Notice the atoms don’t disappear, they just change their identity.

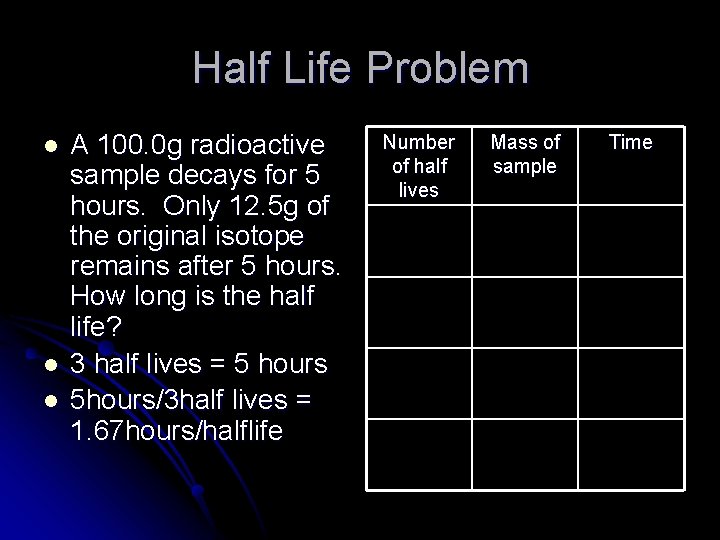
Half Life Problem l l l A 100. 0 g radioactive sample decays for 5 hours. Only 12. 5 g of the original isotope remains after 5 hours. How long is the half life? 3 half lives = 5 hours 5 hours/3 half lives = 1. 67 hours/halflife Number of half lives Mass of sample Time 0 100. 0 g 0 1 50. 0 g 2 25. 0 g 3 12. 5 g 5 hours

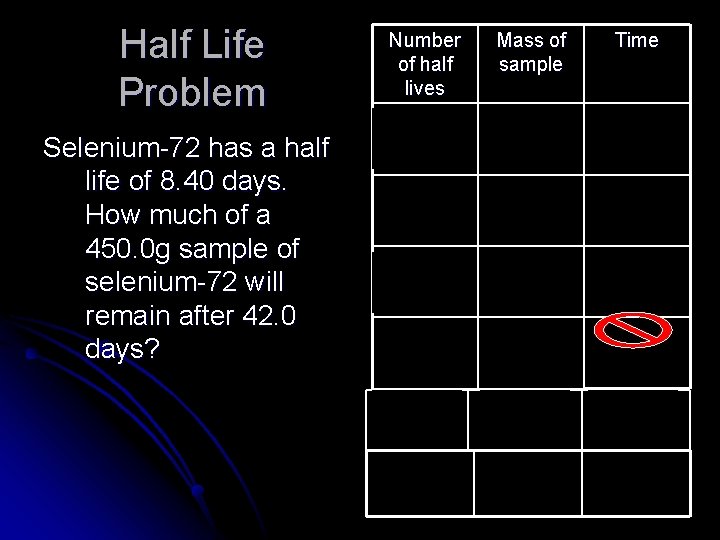
Half Life Problem Selenium-72 has a half life of 8. 40 days. How much of a 450. 0 g sample of selenium-72 will remain after 42. 0 days? Number of half lives Mass of sample Time 0 450. 0 g 0 1 225. 0 g 8. 40 days 2 112. 5 g 16. 8 days 3 56. 25 g 33. 6 days 25. 2 days 4 28. 13 g 33. 6 days 5 14. 06 g 42. 0 days

Radiocarbon Dating Technique l Uses the known half life of C-14 to estimate death of organic matter l Based on the known ratio of C-14 to C-12


Nuclear Fission and Fusion

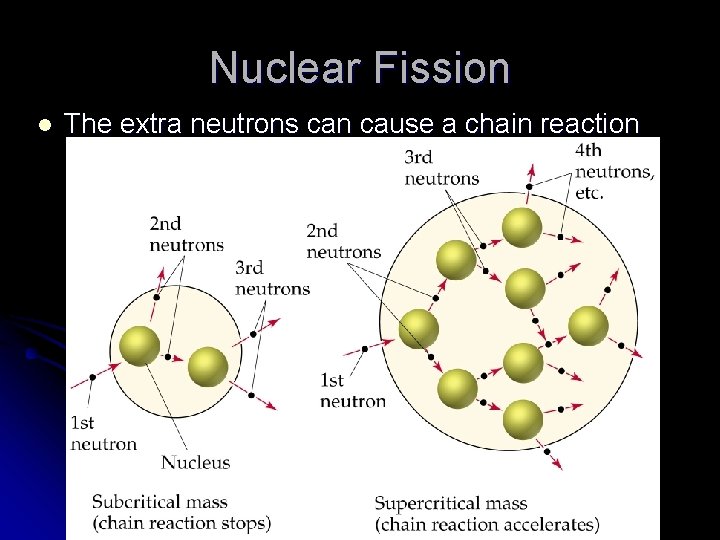
Nuclear Fission l Nuclear fission – one atom’s nucleus splits apart. A neutron strikes a nucleus causing it to split into small pieces l Releases lots of energy. l Extra neutrons are also produced. l

Nuclear Fission l The extra neutrons can cause a chain reaction

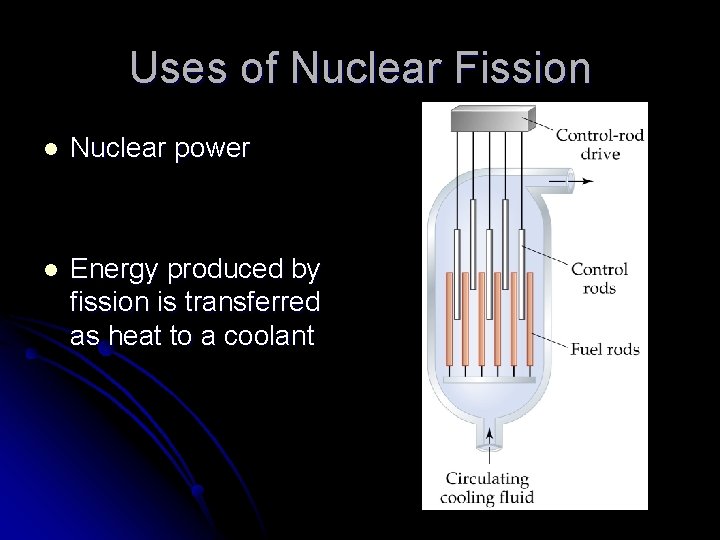
Uses of Nuclear Fission l Nuclear power l Energy produced by fission is transferred as heat to a coolant

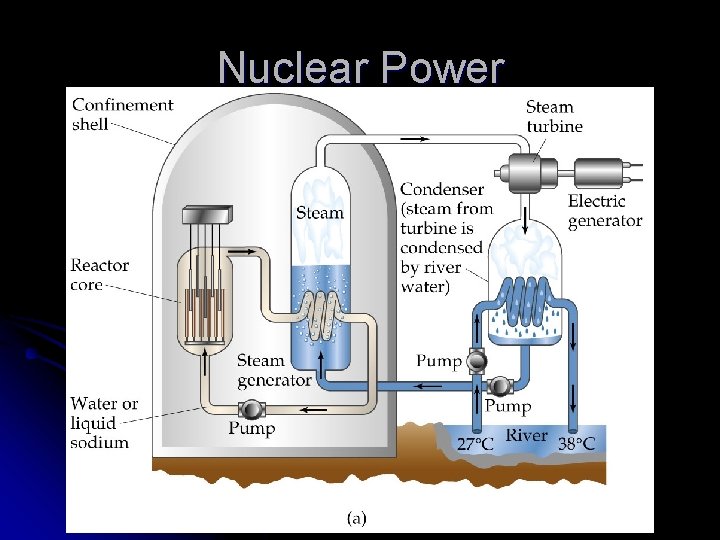
Nuclear Power

Uses of Nuclear Fission Mushroom cloud from Nagasaki

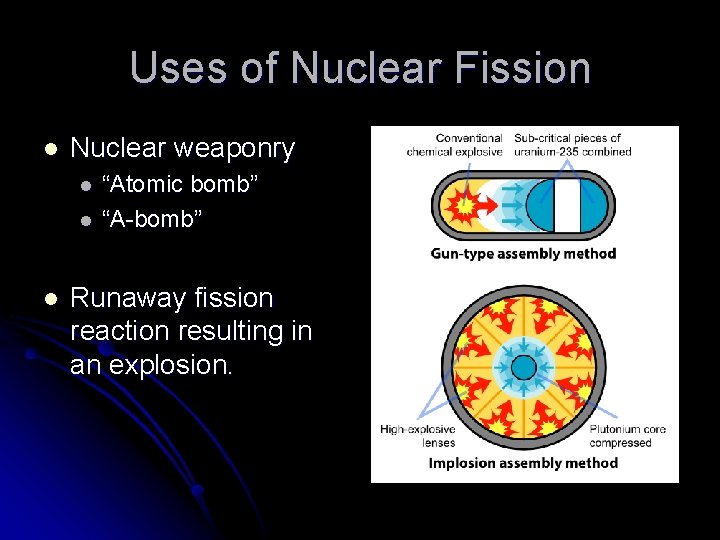
Uses of Nuclear Fission l Nuclear weaponry l l l “Atomic bomb” “A-bomb” Runaway fission reaction resulting in an explosion.


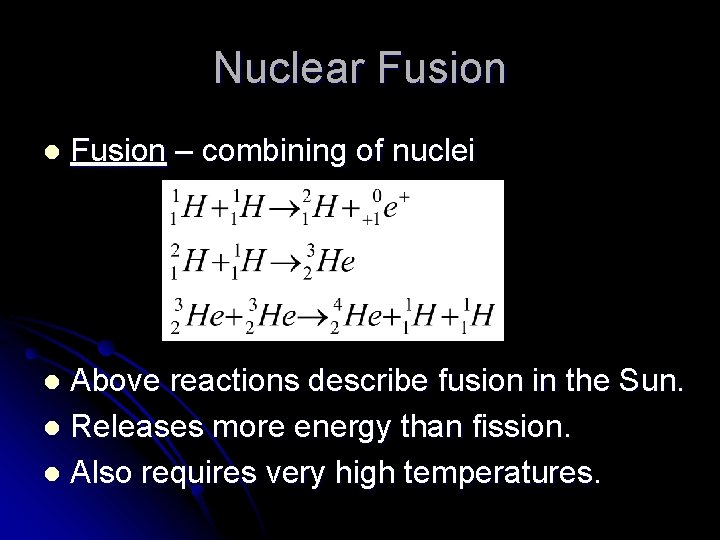
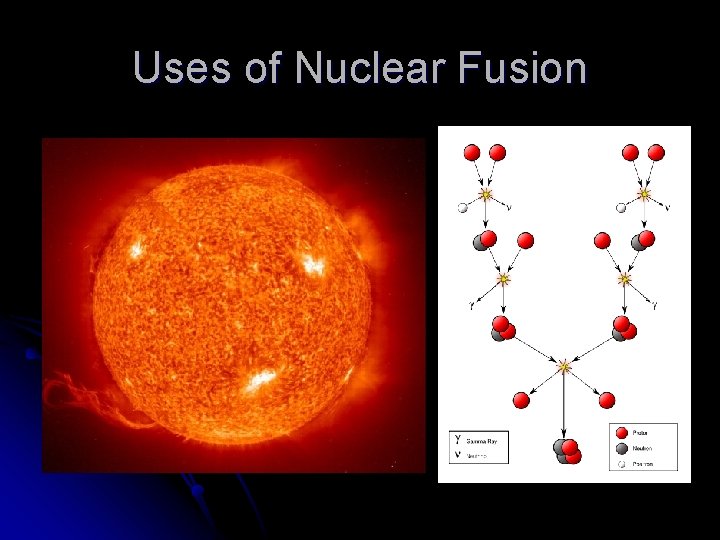
Nuclear Fusion l Fusion – combining of nuclei Above reactions describe fusion in the Sun. l Releases more energy than fission. l Also requires very high temperatures. l

Uses of Nuclear Fusion

Uses of Nuclear Fusion l Fusion weapons l l l Hydrogen bomb “H-bomb” Thermonuclear bomb Uses a fission reaction to start the fusion reaction “A-bomb” is the detonator for an “H-bomb”

Radiation

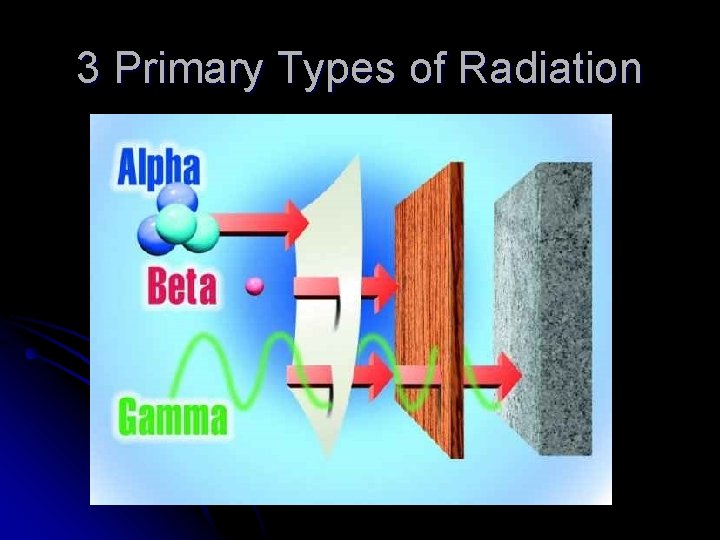
3 Primary Types of Radiation 1. Alpha radiation – α – alpha particles l l 2. Beta radiation – β –beta particles (electrons) l l 3. Low energy Result of alpha decay Low penetration Stop with a sheet of paper Higher energy Result of beta decay Some penetration ability Stop with several sheets of aluminum foil Gamma radiation – γ –high energy gamma rays l l Very high energy Can result from any type of decay Very highly penetrating and damaging Stop with several centimeters of lead or very thick concrete

3 Primary Types of Radiation

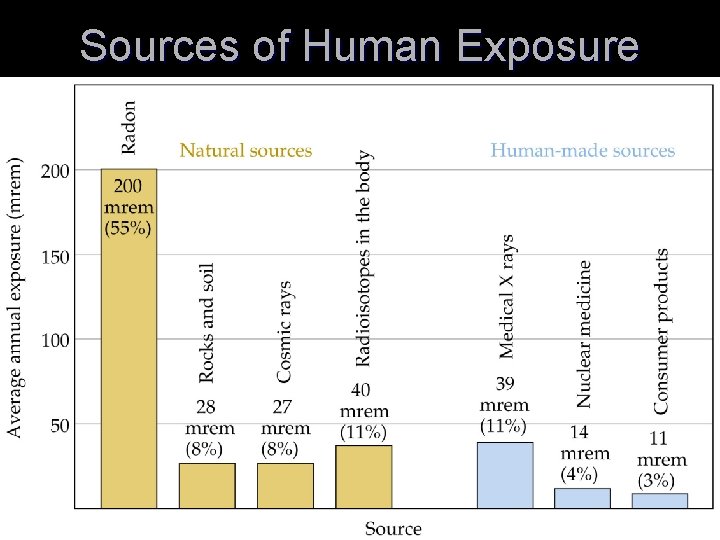
Sources of Human Exposure
 Weird restaurants around the world
Weird restaurants around the world Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Chernobyl webquest answer key
Chernobyl webquest answer key Chemistry
Chemistry Application of nuclear chemistry
Application of nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Effective nuclear charge trend
Effective nuclear charge trend Chapter 24 nuclear chemistry answer key
Chapter 24 nuclear chemistry answer key Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Anatomy of a wave
Anatomy of a wave 25 m/s
25 m/s T half life formula
T half life formula Nuclear fusion in real life
Nuclear fusion in real life Chapter 21 review nuclear chemistry
Chapter 21 review nuclear chemistry Nuclear chemistry
Nuclear chemistry Nitrogen-13 decay equation
Nitrogen-13 decay equation Applications of nuclear chemistry
Applications of nuclear chemistry Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Aj my weird school
Aj my weird school Double object verbs
Double object verbs Azusa revival 1906
Azusa revival 1906 Nil nisi bonum pronunciation
Nil nisi bonum pronunciation Comunitatea amish
Comunitatea amish Mobius cellotape
Mobius cellotape Tudor food and drink
Tudor food and drink Cloud watching sound weird but hang adores it
Cloud watching sound weird but hang adores it Weird wild wacky personality disorders
Weird wild wacky personality disorders Comparative degree of fat
Comparative degree of fat Weird true and freaky animal planet
Weird true and freaky animal planet