Nuclear chemistry Nucleus decay Nuclear chemistry How do






- Slides: 10

Nuclear chemistry Nucleus decay

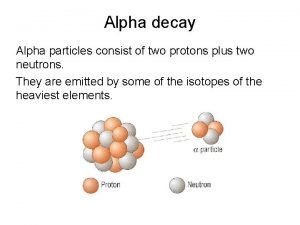
Nuclear chemistry • How do you write equations with different types of nuclear decay? • It all depends on the type of decay. • The symbols should represent what happens to the nucleus. • Alpha • Beta • Gamma • Electron capture • Positron emission

Nuclear chemistry • Draw the formula for the following radioactive decays. • Polonium-210 goes through alpha decay. • Carbon-14 goes through beta decay. • Carbon-11 goes through positron emission. • Rubidium-81 goes through electron capture.

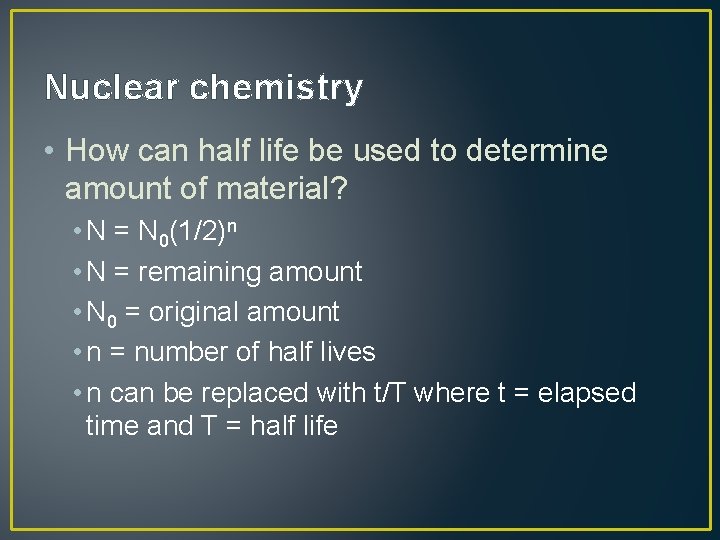
Nuclear chemistry • How can half life be used to determine amount of material? • N = N 0(1/2)n • N = remaining amount • N 0 = original amount • n = number of half lives • n can be replaced with t/T where t = elapsed time and T = half life

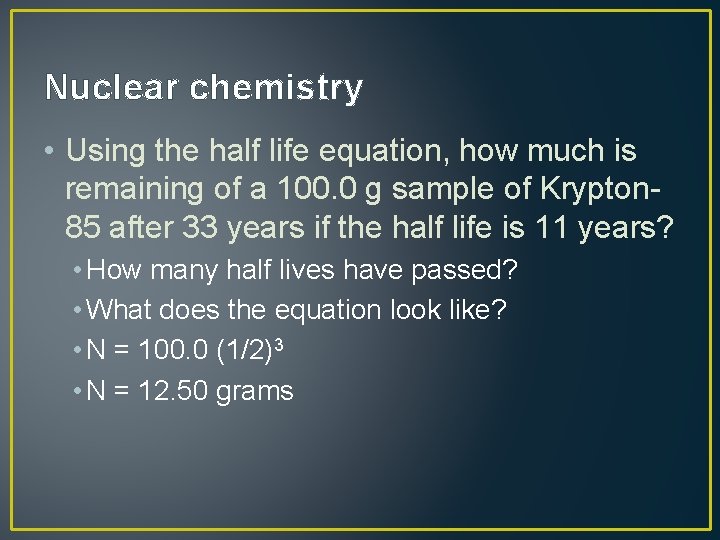
Nuclear chemistry • Using the half life equation, how much is remaining of a 100. 0 g sample of Krypton 85 after 33 years if the half life is 11 years? • How many half lives have passed? • What does the equation look like? • N = 100. 0 (1/2)3 • N = 12. 50 grams

Nuclear chemistry • The half life of polonium-218 is 3. 0 minutes. How long before a 20. 0 g sample would decay to 1. 00 grams? • What equation should we use? • How can you solve for an exponent? • 13 minutes.

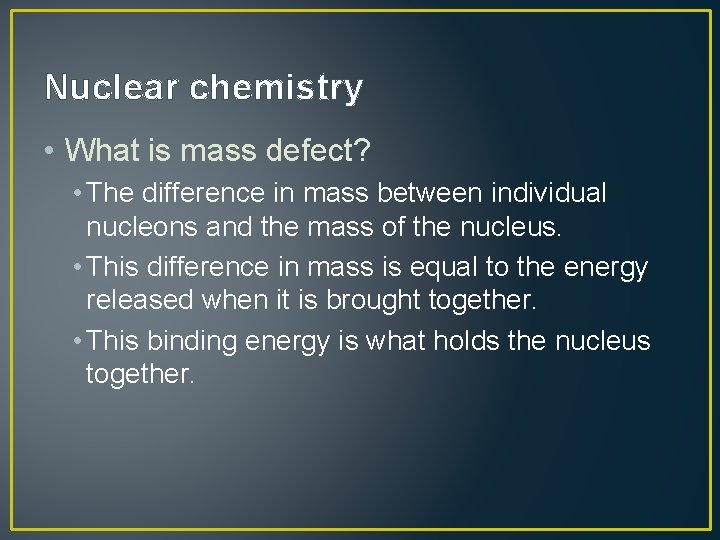
Nuclear chemistry • What is mass defect? • The difference in mass between individual nucleons and the mass of the nucleus. • This difference in mass is equal to the energy released when it is brought together. • This binding energy is what holds the nucleus together.

Nuclear chemistry • How can we use E = mc 2 to find the binding energy of an atom? • Mass defect is the mass of the isotope minus the mass of the added nucleons. • Proton = 1. 007825 amu • Neutron = 1. 008665 amu • 1 amu = 1. 660540 x 10 -27 kg • c = 3. 00 x 108 m/s

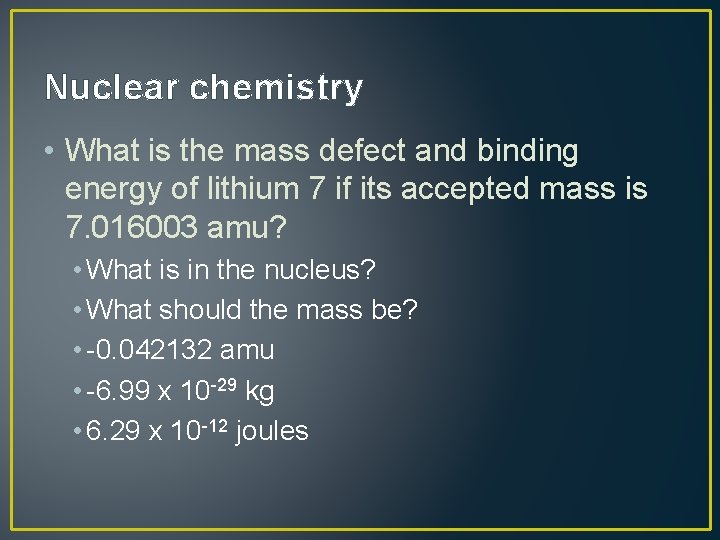
Nuclear chemistry • What is the mass defect and binding energy of lithium 7 if its accepted mass is 7. 016003 amu? • What is in the nucleus? • What should the mass be? • -0. 042132 amu • -6. 99 x 10 -29 kg • 6. 29 x 10 -12 joules

Nuclear chemistry • What are the uses of radiation? • Fission and fusion (theoretical) energy reactors. • Radioactive isotope tracers. • Treatment of cancer. • PET scans.