Nuclear Chemistry The Nucleus A The Nucleus Made









- Slides: 31

Nuclear Chemistry The Nucleus

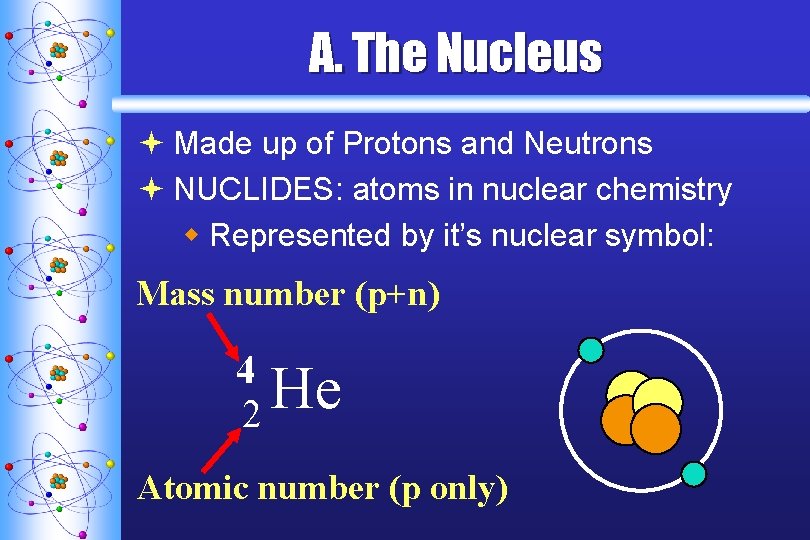
A. The Nucleus ª Made up of Protons and Neutrons ª NUCLIDES: atoms in nuclear chemistry w Represented by it’s nuclear symbol: Mass number (p+n) 4 He 2 Atomic number (p only)

A. The Nucleus ª Nuclear Reaction: w a reaction that affects the nucleus of an atom w Gives off large amts of energy (mass of product is less than mass of reactant) w Increases stability ª Transmutation: w One element becomes another.

Nuclear Chemistry Radioactive Decay

A. Radioactive Decay ª The spontaneous disintegration of a nucleus into a slightly lighter nucleus w Emits particles, electromagnetic (EM) radiation, or both ª This emission is referred to as: NUCLEAR RADIATION ª Radioactive nuclide: an unstable nucleus that undergoes radioactive decay Ex: Uranium

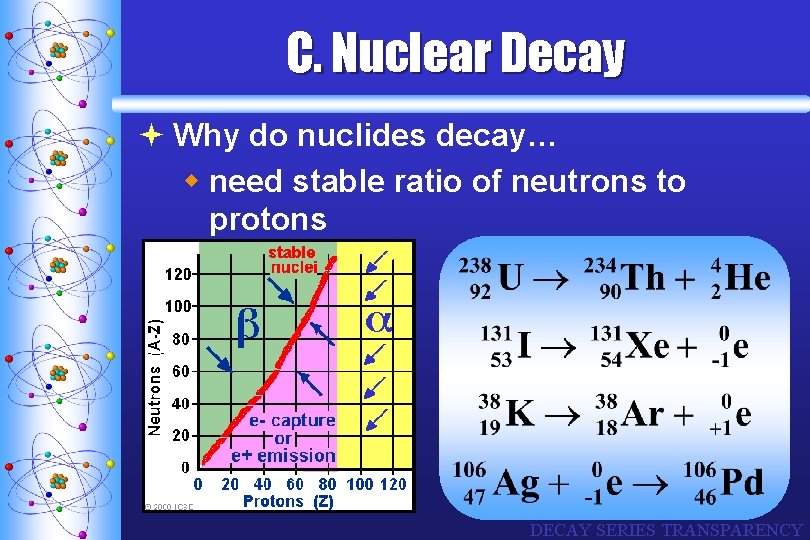
C. Nuclear Decay ª Why do nuclides decay… w need stable ratio of neutrons to protons DECAY SERIES TRANSPARENCY

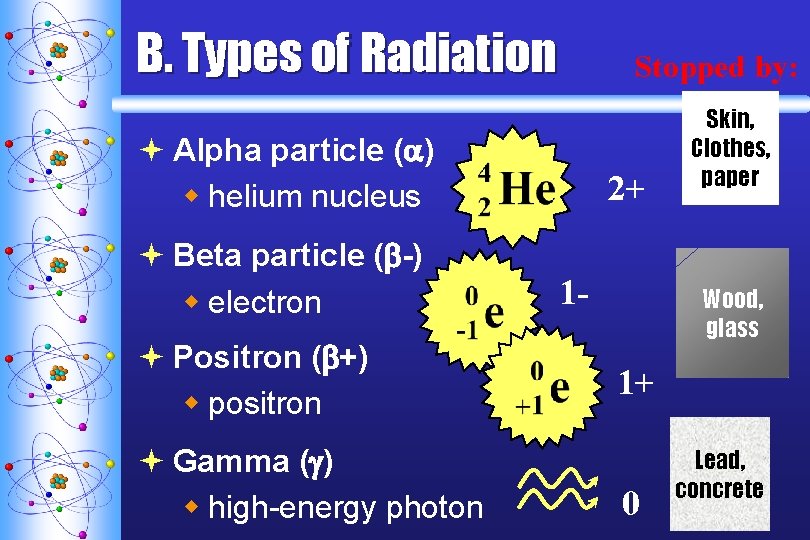
B. Types of Radiation Stopped by: ª Alpha particle ( ) w helium nucleus ª Beta particle ( -) w electron ª Positron ( +) w positron ª Gamma ( ) w high-energy photon 2+ 1 - Skin, Clothes, paper Wood, glass 1+ 0 Lead, concrete

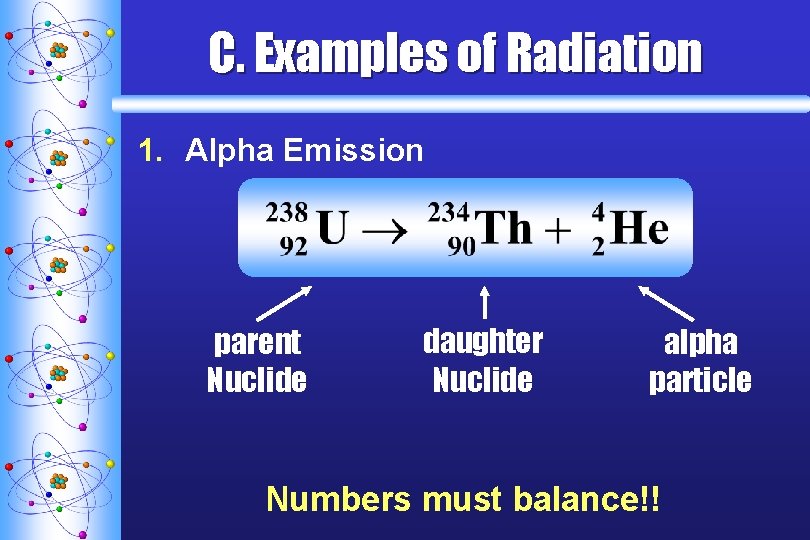
C. Examples of Radiation 1. Alpha Emission parent Nuclide daughter Nuclide alpha particle Numbers must balance!!

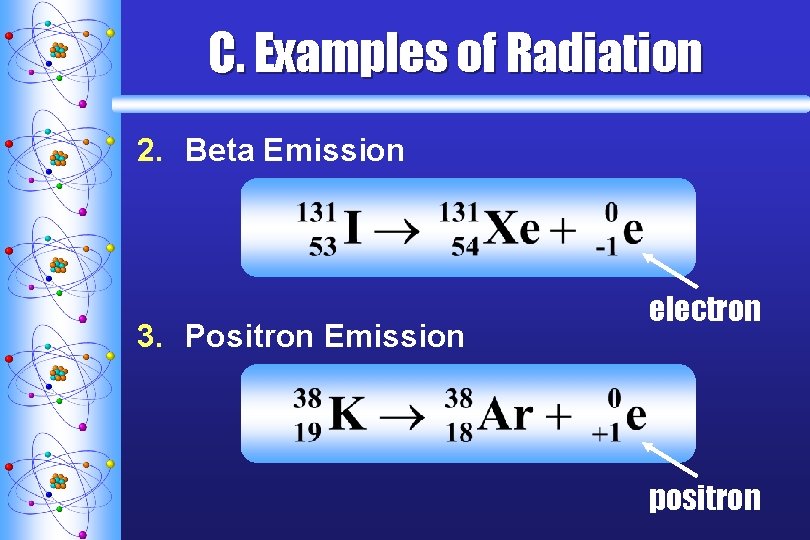
C. Examples of Radiation 2. Beta Emission 3. Positron Emission electron positron

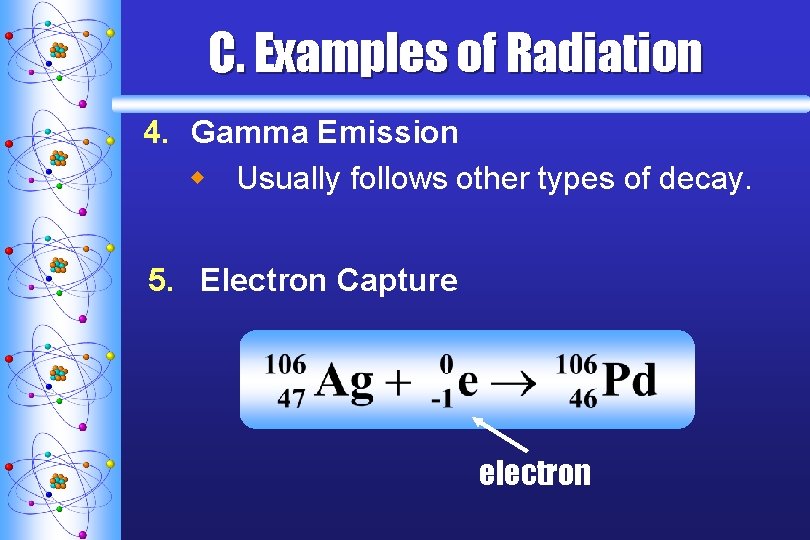
C. Examples of Radiation 4. Gamma Emission w Usually follows other types of decay. 5. Electron Capture electron

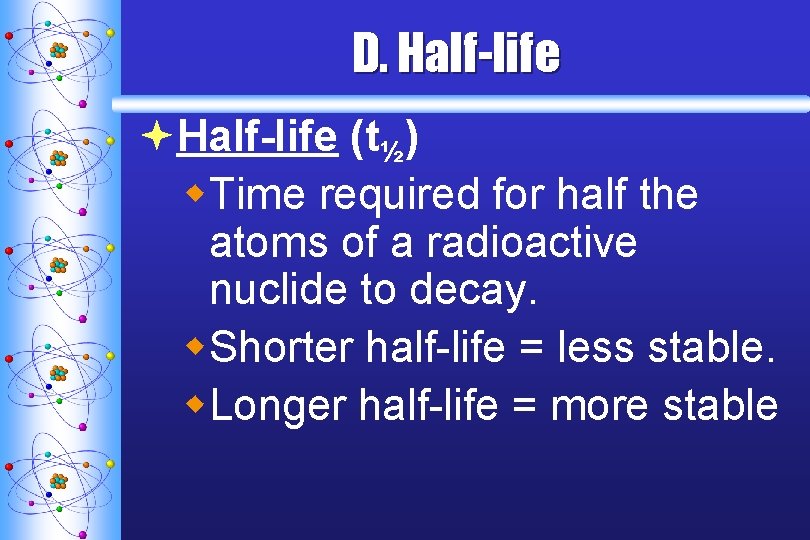
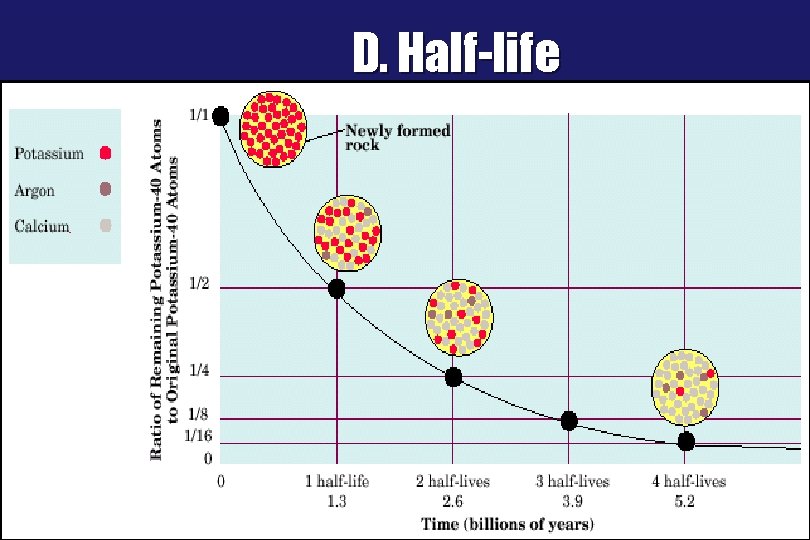
D. Half-life ªHalf-life (t½) w. Time required for half the atoms of a radioactive nuclide to decay. w. Shorter half-life = less stable. w. Longer half-life = more stable

D. Half-life

E. Calculations Carbon 11 has a half-life of 20 minutes. After 3 hours pass, how many half-lives have passed? ª t 1/2 = 20 minutes ª Total time: 180 minutes 180 min 20 min = 9 half-lives

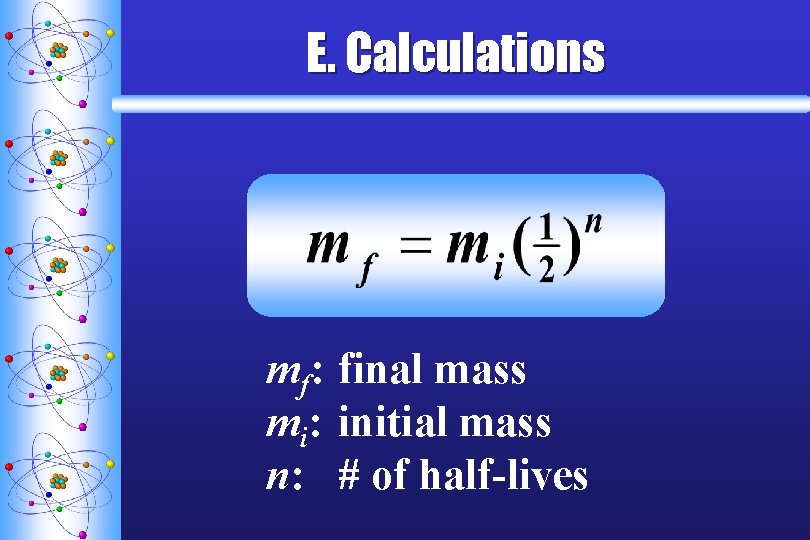
E. Calculations mf: final mass mi: initial mass n: # of half-lives

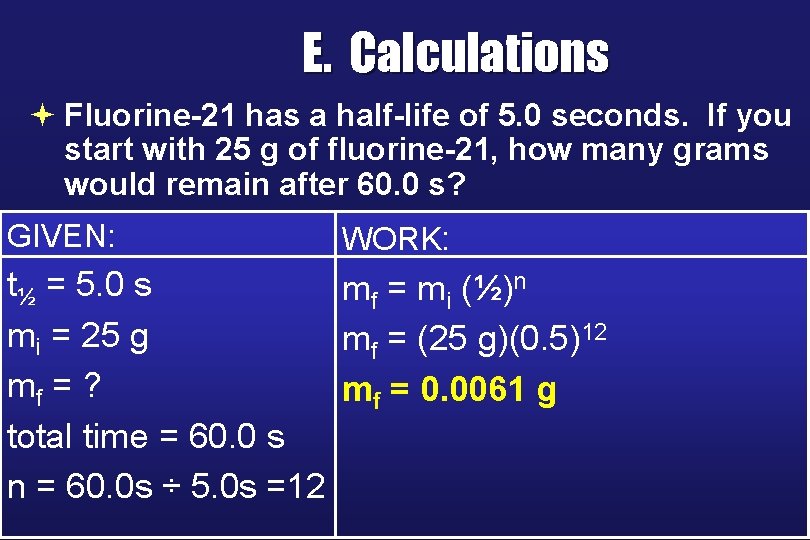
E. Calculations ª Fluorine-21 has a half-life of 5. 0 seconds. If you start with 25 g of fluorine-21, how many grams would remain after 60. 0 s? GIVEN: WORK: t½ = 5. 0 s mf = mi (½)n mi = 25 g mf = (25 g)(0. 5)12 mf = ? mf = 0. 0061 g total time = 60. 0 s n = 60. 0 s ÷ 5. 0 s =12

Nuclear Chemistry Fission & Fusion

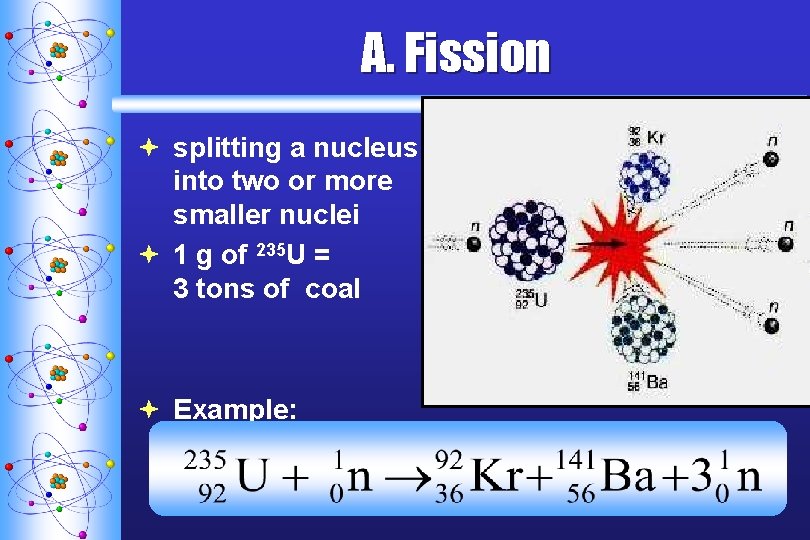
A. F ission ª splitting a nucleus into two or more smaller nuclei ª 1 g of 235 U = 3 tons of coal ª Example:

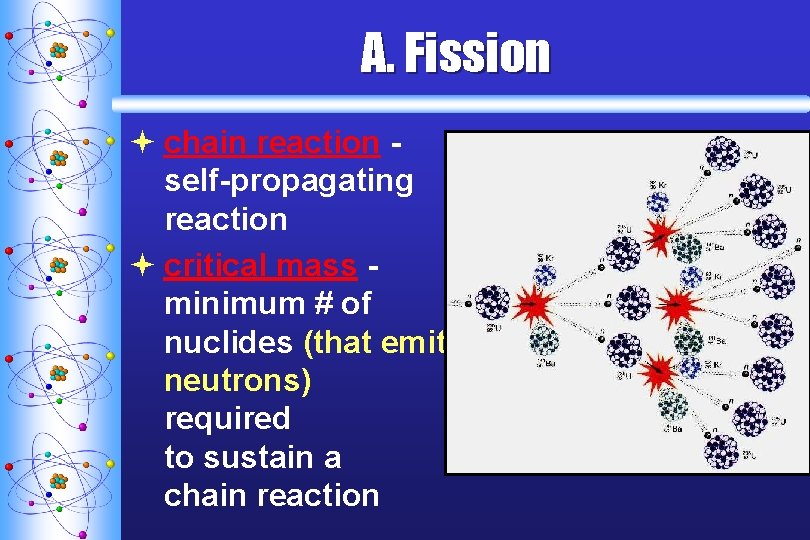
A. F ission ª chain reaction self-propagating reaction ª critical mass minimum # of nuclides (that emit neutrons) required to sustain a chain reaction

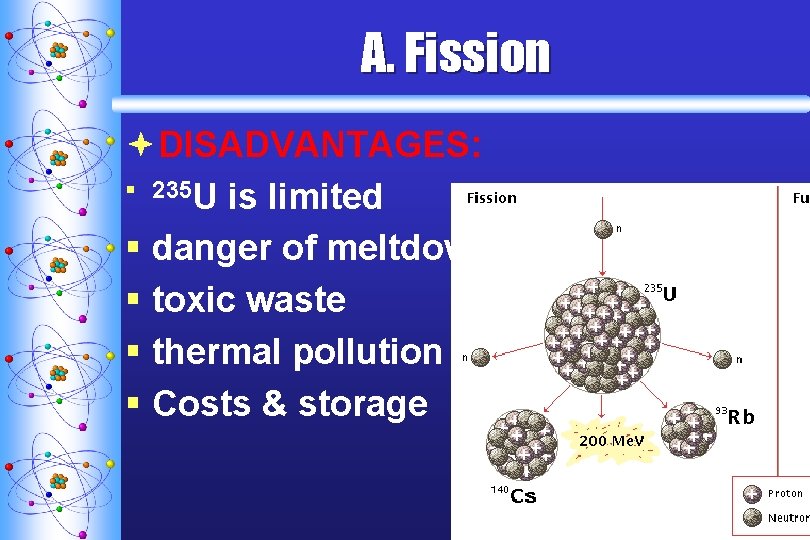
A. F ission ªDISADVANTAGES: § 235 U is limited § danger of meltdown § toxic waste § thermal pollution § Costs & storage

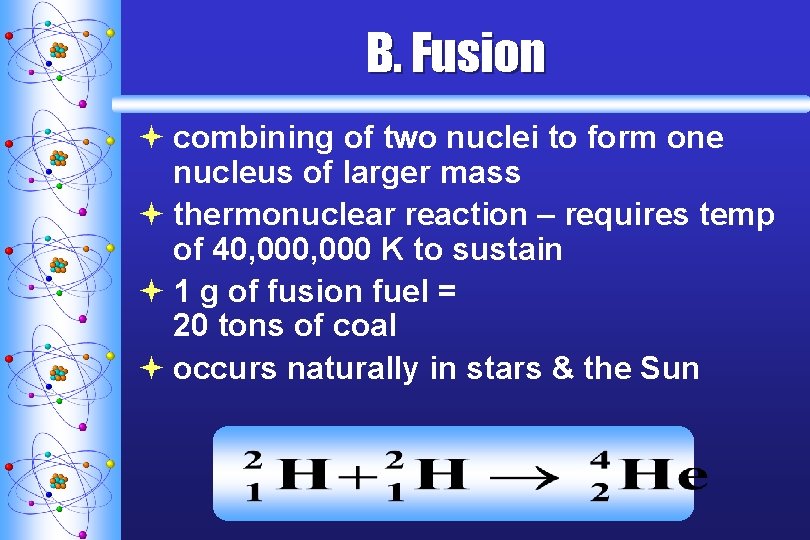
B. Fusion ª combining of two nuclei to form one nucleus of larger mass ª thermonuclear reaction – requires temp of 40, 000 K to sustain ª 1 g of fusion fuel = 20 tons of coal ª occurs naturally in stars & the Sun

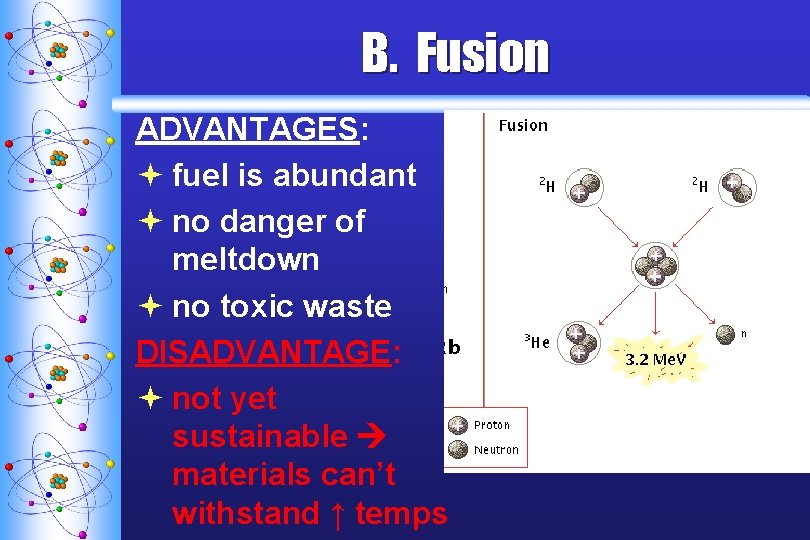
B. Fusion ADVANTAGES: ª fuel is abundant ª no danger of meltdown ª no toxic waste DISADVANTAGE: ª not yet sustainable materials can’t withstand ↑ temps

Nuclear Chemistry Applications

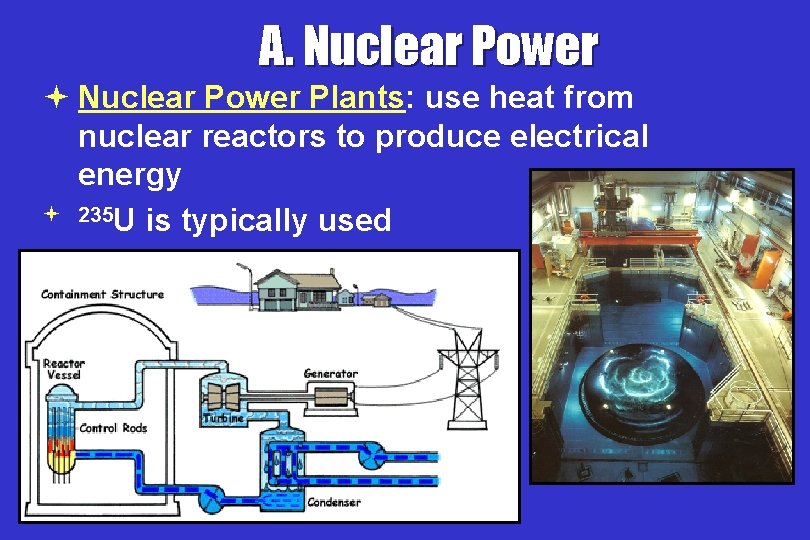
A. Nuclear Power ª Nuclear Power Plants: use heat from nuclear reactors to produce electrical energy ª 235 U is typically used

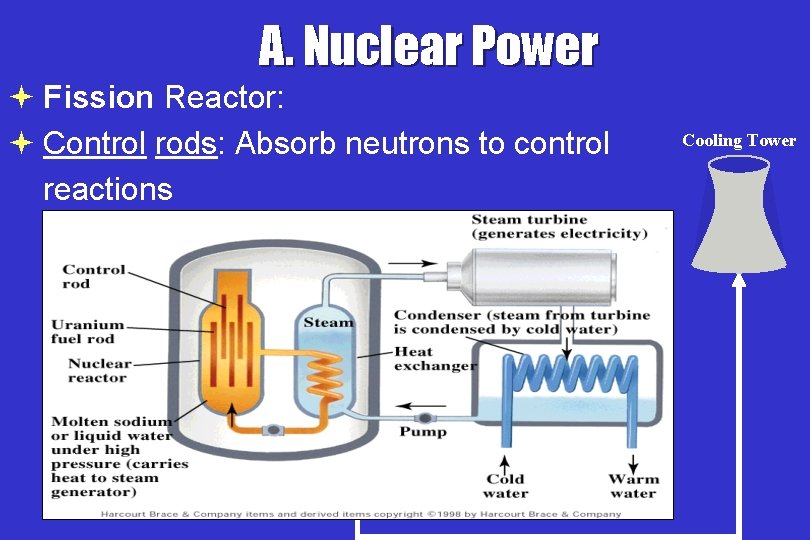
A. Nuclear Power ª Fission Reactor: ª Control rods: Absorb neutrons to control reactions * Cooling Tower

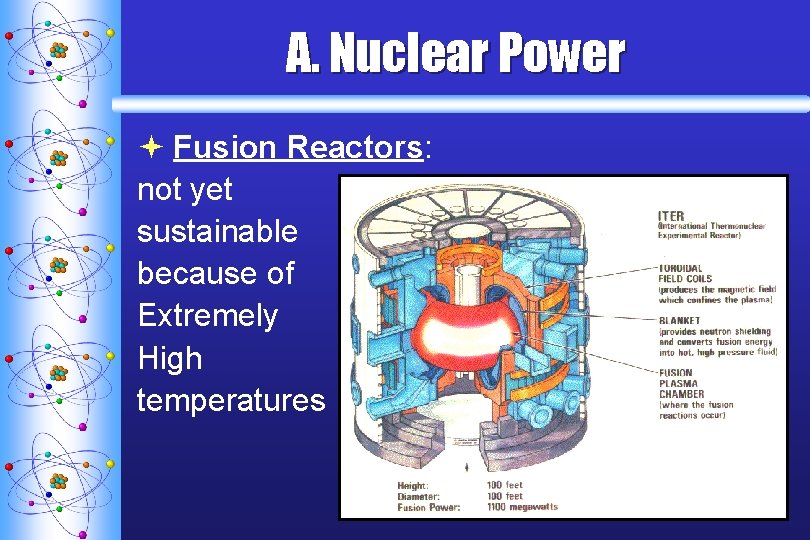
A. Nuclear Power ª Fusion Reactors: not yet sustainable because of Extremely High temperatures

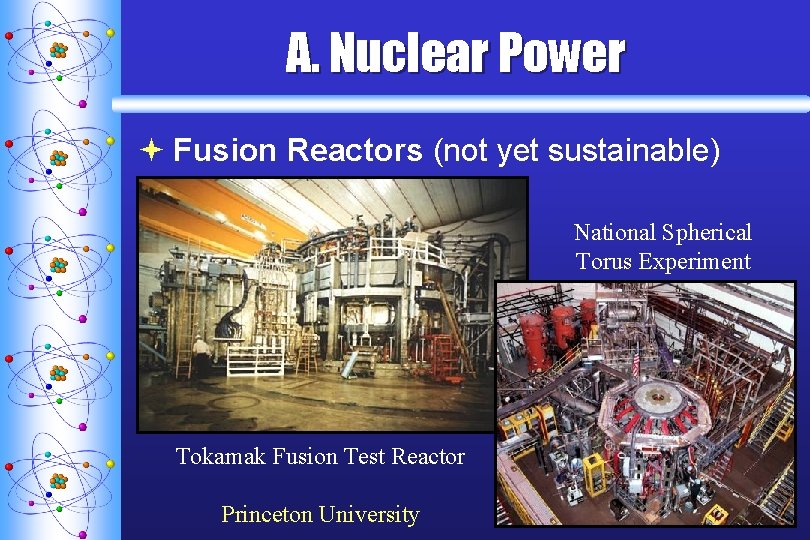
A. Nuclear Power ª Fusion Reactors (not yet sustainable) National Spherical Torus Experiment Tokamak Fusion Test Reactor Princeton University

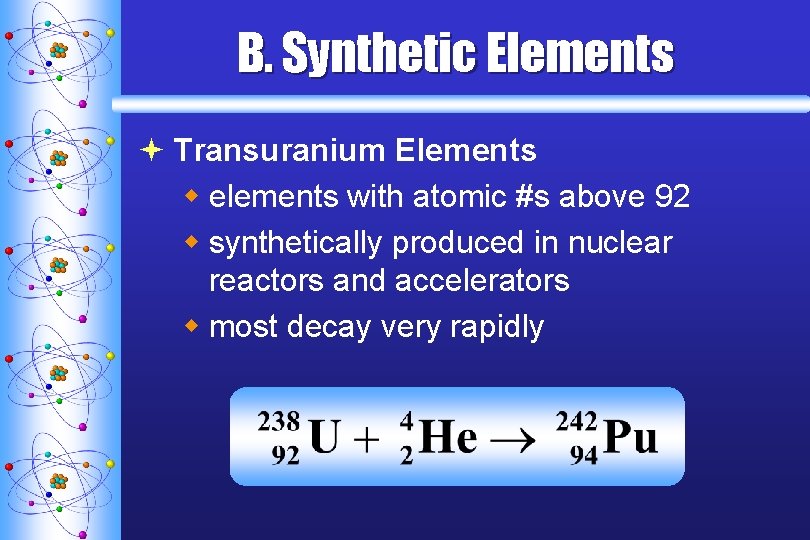
B. Synthetic Elements ª Transuranium Elements w elements with atomic #s above 92 w synthetically produced in nuclear reactors and accelerators w most decay very rapidly

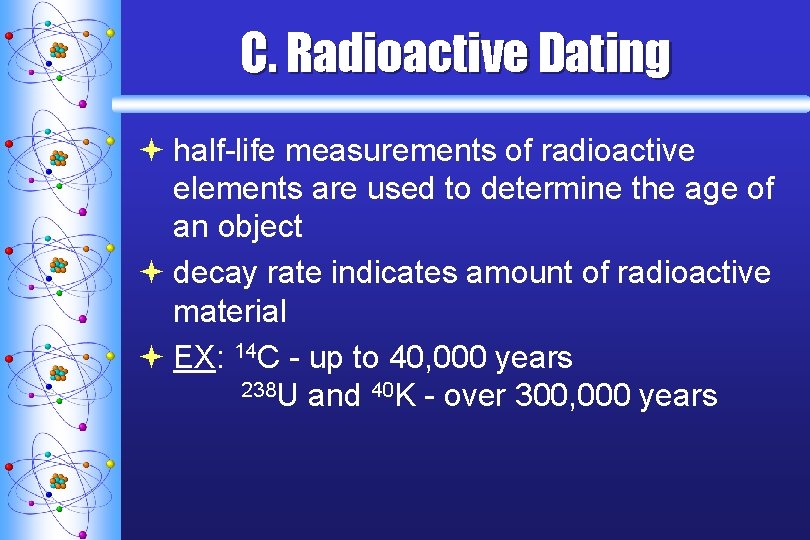
C. Radioactive Dating ª half-life measurements of radioactive elements are used to determine the age of an object ª decay rate indicates amount of radioactive material ª EX: 14 C - up to 40, 000 years 238 U and 40 K - over 300, 000 years

D. Nuclear Medicine ª Radioisotope Tracers w absorbed by specific organs and used to diagnose diseases ª Radiation Treatment w larger doses are used to kill cancerous cells in targeted organs w internal or external radiation source Radiation treatment using -rays from cobalt-60.

E. Nuclear Weapons ª Atomic Bomb w chemical explosion is used to form a critical mass of 235 U or 239 Pu w fission develops into an uncontrolled chain reaction ª Hydrogen Bomb w chemical explosion fission fusion w fusion increases the fission rate w more powerful than the atomic bomb

F. Others ª Food Irradiation w Gamma ( ) radiation is used to kill bacteria ª Radioactive Tracers w explore chemical pathways w trace water flow w study plant growth, photosynthesis ª Consumer Products w ionizing smoke detectors - 241 Am